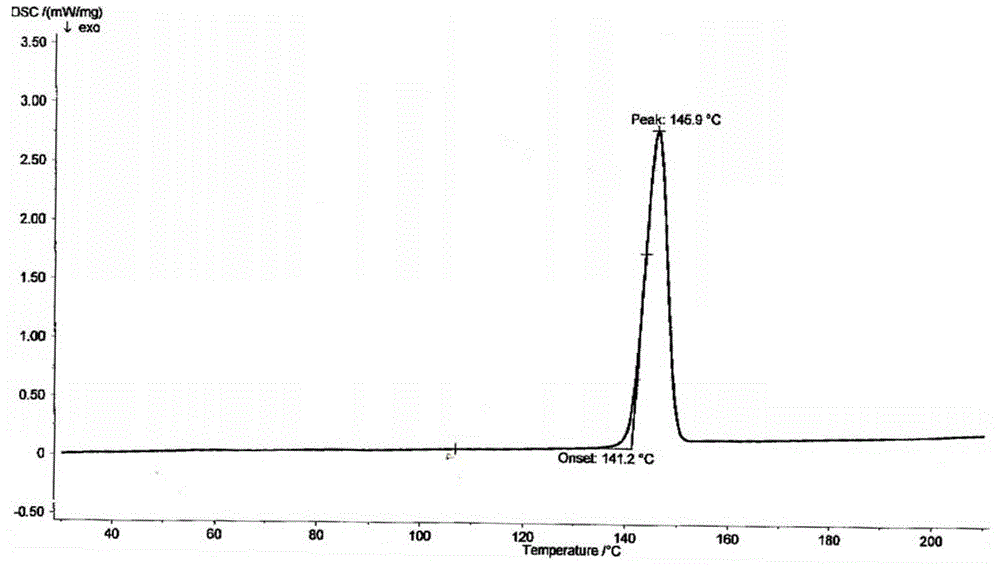
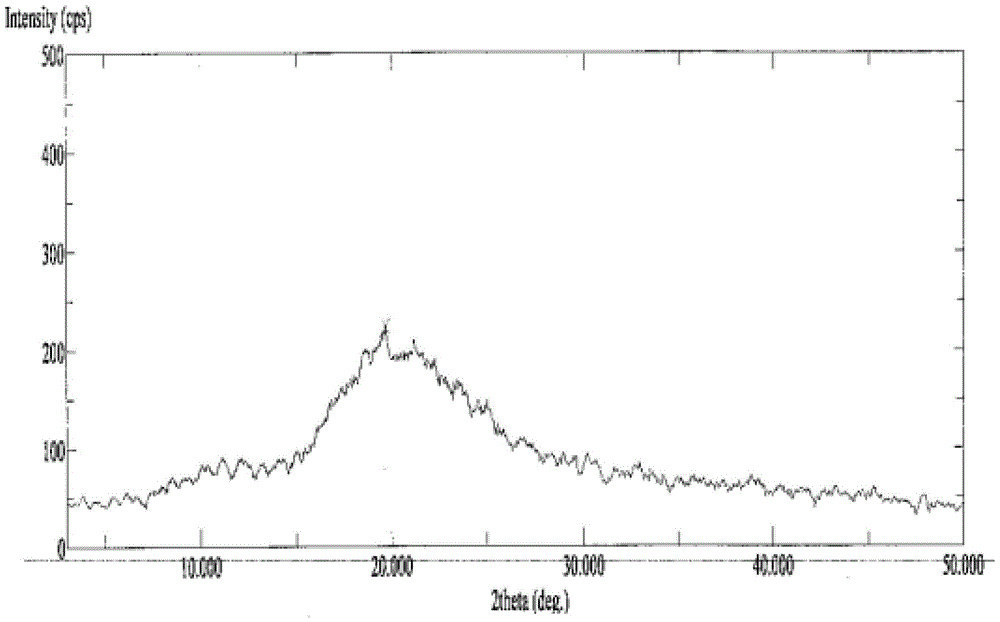
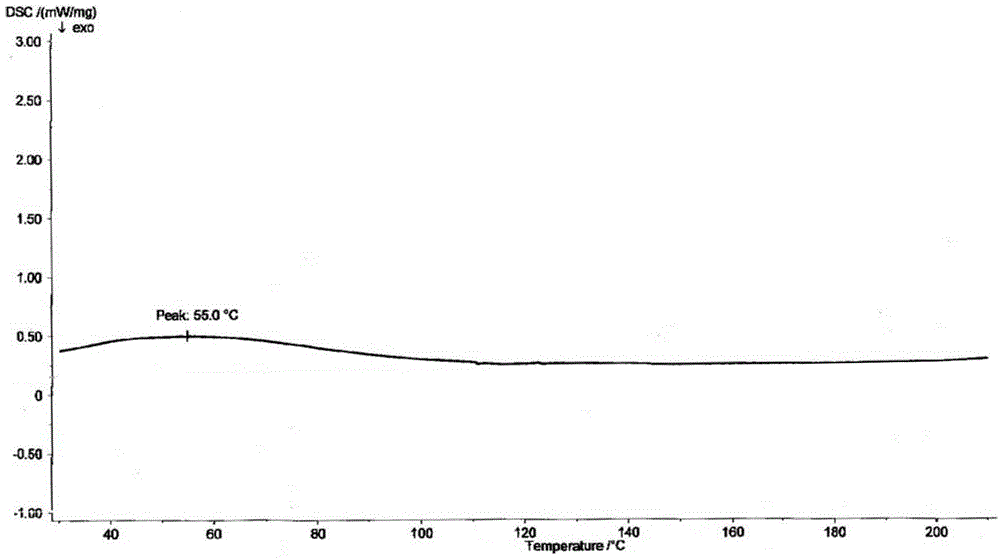
A powder of dronedarone or its salt and pharmaceutical composition prepared therefrom
A technology of dronedarone and its composition, which is applied in the field of antiarrhythmic drugs, can solve problems such as the dissolution rate of dronedarone hydrochloride, difficulty in controlling the properties of mixture excipients, drug release and stability effects, etc., to facilitate the preparation operation , good dissolution effect, the effect of improving dissolution rate and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Based on the weight of dronedarone hydrochloride, add soybean lecithin, Tween-20, Tween-80, and macrogol glyceride laurate in the weight ratio shown in Table 1, and dissolve in the phosphate buffer of pH 4.5 to prepare into a solution containing dronedarone hydrochloride 2mg / mL, incubated at 37°C for 2 hours, then diluted the solution 10 times with neutral phosphate buffer, the final pH of the solution was 6.8, and incubated at 37°C for 2 hours, and passed through a 5 μm Filter the solution, use a UV spectrophotometer to measure the absorbance value A of the solution at a wavelength of 217nm; take another phosphate buffer solution with pH 4.5 to make a solution containing dronedarone hydrochloride 2mg / mL, and keep it warm at 37°C for 2 hours. Then the solution is diluted 10 times with the phosphate buffer solution of pH 4.5, the final pH of the solution is 4.5, and after being incubated at 37°C for 2 hours, it is filtered through a filter of 5 μm. Absorbance value B, ca...
Embodiment 2
[0045] Embodiment 2 dronedarone hydrochloride powder
[0046] Disperse 42.6 g of dronedarone hydrochloride and 4 g of soybean lecithin in 500 mL of 70% ethanol, and spray dry it with a spray dryer to obtain a powder. (Wherein, the weight of soybean lecithin accounts for about 8.6% of the powder weight)
[0047] Spray drying conditions: spray dryer; nitrogen flow pressure: 600L / h; inlet air temperature: 90°C; outlet air temperature: 43°C; feed rate: 5mL / min; needle pass frequency: 15 seconds / time.
Embodiment 3
[0048] Example 3 Dronedarone Hydrochloride Powder
[0049] Disperse 42.6 g of dronedarone hydrochloride and Tween-804 g in 500 mL of absolute ethanol, and spray dry it with a spray dryer to obtain a powder. (Wherein, the weight of Tween-80 accounts for about 8.6% of powder weight)
[0050] Spray drying conditions: spray dryer; nitrogen flow pressure: 1200L / h; air inlet temperature: 80°C; air outlet temperature: 32°C; feed rate: 15mL / min; needle pass frequency: 30 seconds / time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com