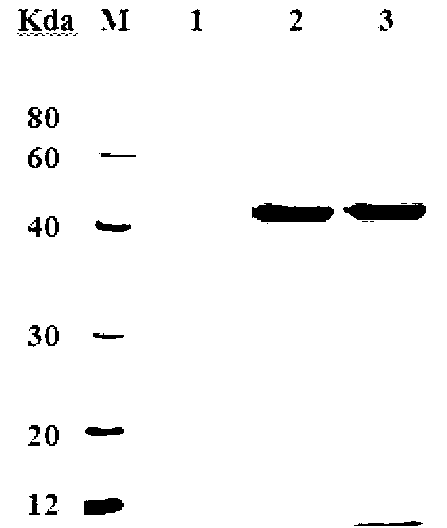Application of protein and composition thereof in preparation of product used for preventing, diagnosing or treating EHEC (Enterohemorrhagic Escherichia Coli) infection
A composition and protein technology, applied in the direction of peptide/protein components, immunoglobulins from serum, medical preparations containing active ingredients, etc., can solve the problem of incomplete annotation of genome functions, unclarified functions of virulence factors, and encoded protein functions Issues such as not being confirmed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1, the acquisition of protein and its composition and its recognition to infected EHEC serum
[0066] 1. Amplification of the target gene
[0067] According to the target gene nucleotide sequence of EHEC O157:H7 published by GenBank, primers were designed and synthesized by introducing 6×His tag (CACCACCACCACCACCAC) into the downstream primers considering the requirements of protein purification.
[0068] Table 1 is the primer name, sequence and restriction site
[0069]
[0070] Using the extracted EHEC O157:H7 genome as a template, the upstream and downstream primers of Z0390 and the upstream and downstream primers of Z4191 were used for PCR amplification respectively, and a PCR product of 1137bp and a PCR product of 597bp were obtained.
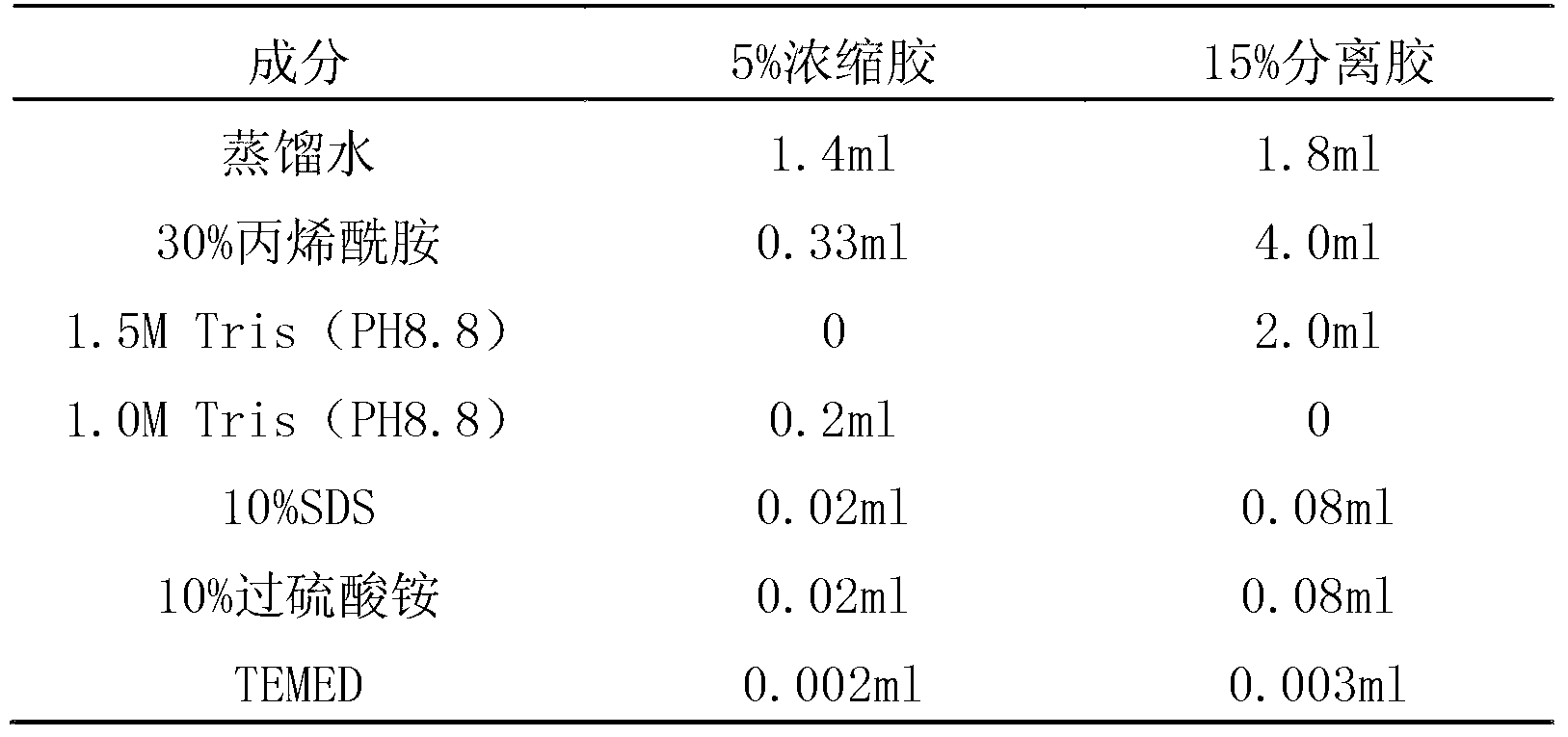
[0071] The 50 μl amplification reaction system required for the above PCR amplification is: 1 μl of upstream and downstream primers, 1 μl of template genomic DNA, 4 μl of dNTP, 5 μl of 10×Buffer, 1 μl of Taq enzyme, and ...
Embodiment 2
[0103] Embodiment 2, the immunogenicity of protein or protein composition and the protective effect to EHEC infection
[0104] Female BALB / c mice 14-16g were randomly divided into 2 groups, 10 in each group, and the following experiments were repeated three times:
[0105] Z0390 immune group: the purified recombinant protein Z0390-HIS obtained in Example 1 was immunized by intraperitoneal route as antigen;
[0106] Z4191 immunization group: the purified recombinant protein Z4191-HIS obtained in Example 1 was immunized by intraperitoneal route as antigen;
[0107] Z0390 / Z4191 mixed immunization group I: intraperitoneal route immunization with purified recombinant protein Z0390-HIS and purified recombinant protein Z4191-HIS obtained in Example 1 as immunogens; mass ratio of purified recombinant protein Z0390-HIS and purified recombinant protein Z4191-HIS 1:1;
[0108] Z0390 / Intimin mixed immunization group II: intraperitoneal route immunization of the purified recombinant prot...
Embodiment 3
[0127] Embodiment 3, the preparation and application of the antibody or antibody group of anti-protein antigen
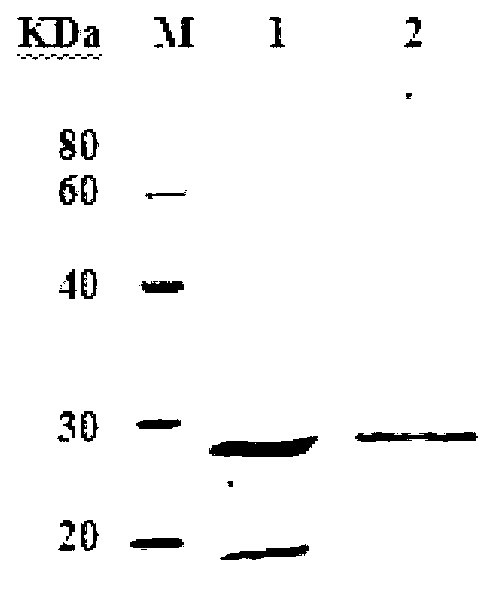
[0128] 1. Preparation and identification of protein antigen antiserum
[0129] The purified recombinant protein Z0390-HIS and the purified recombinant protein Z4191-HIS obtained in Example 1 with a concentration of 1 mg / ml and a purity of more than 95% were used as antigens to immunize big-eared white rabbits (male, 2.5 kg). Respectively in the fourth week (25μg / monkey) and eighth week (50μg / bird) booster immunization. When the titer (ELISA detection) reaches and stabilizes at 1:10 5 As mentioned above, blood was collected from the heart of Daerbai, and the serum was separated by centrifugation at 5000g for 10 minutes, and the serum was filtered with a 2 μm disposable filter (Millipore). The filtered serum was purified by affinity chromatography (Protein A Hitrap, GE Company, operation Follow the instructions), collect 1.2 column volumes for anti-Z0390 antibody (p...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap