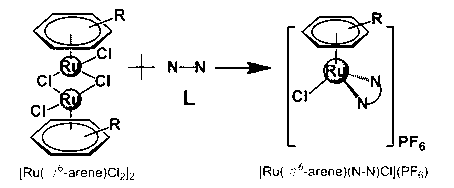
Aryl ruthenium-beta-carboline complex and its preparation method and application
A complex, aryl ruthenium technology, applied in aryl ruthenium-β-carboline complexes and its field, can solve the problems of limiting the actual curative effect and scope of application, side effects, difficult to break platinum drugs, etc., and achieve toxicity reduction , highlight the activity, increase the effect of water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of Ligand L1:
[0041] Add 2-pyridinecarbaldehyde (535 mg, 5.00 mmol), tryptamine (810 mg, 5.00 mmol) and 200 mL of anhydrous anisole into a 500 mL three-necked flask, and heat slowly to 155 °C (about 2 hours). 1.20 g of 10% Pd / C was added, and the reflux reaction was continued for 22 h at 155 °C. Stop the reaction, filter the reaction mixture while hot, and remove most of the anisole in the filtrate by rotary evaporation. Add about 20 mL of ethanol to the filtrate, and let stand to precipitate a yellow precipitate. Filter naturally, wash with ethanol 2-3 times, and dry in vacuo to obtain 1.050 g of yellow powder solid with a yield of 86%.
[0042] Elemental Analysis C 16 h 11 N 3 (Molecular weight 245.2), theoretical value: C 78.35%, H 4.52%; N 17.13%; experimental value: C 78.24%, H 4.53%, N 17.22%. ESI-MS: [M+H] + Theoretical value: m / z = 246.10; Experimental value: m / z = 246.35.
[0043] The structural formula of ligand L1 is:
[0044]
...
Embodiment 2
[0088] Example 2 Inhibition test of Ru(II) complexes on CDK1
[0089] The inhibition of CDK1 activity by the complexes was determined using the ADP-Glo® Kinase Detection Kit from Promega, USA. The kit works by detecting ADP formed in a kinase reaction; ADP is converted to ATP, which is then converted to light by Ultra-Glo® luciferase. The luminescence signal is positively correlated with the activity of the kinase. Purified recombinant CDK / Cyclin B protein was purchased from Millipore, USA. The specific operation steps for activity detection are as follows: in a reaction system with a total volume of 5 μL, add CDK1 / cyclin B (1 ng) and the compound to be tested (0.1 – 50 μM). This reaction system contains 0.2 μg / μL substrate histone, 50 μM ATP, buffer solution components are 8 mM MOPS, pH 7.0, 0.2 mM EDTA, 4 mM magnesium chloride, 50 μM DTT), and incubated at 25 °C for 60 minute. After adding ADP-Glo™ reagent (incubate for 40 minutes) and kinase detection reagent (incubate ...
Embodiment 3
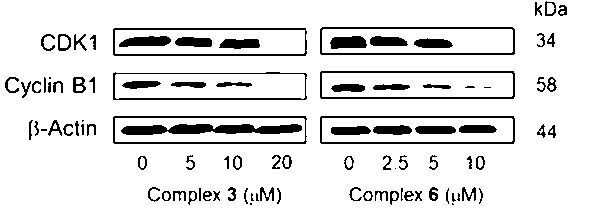
[0092] Example 3 Down-regulation of CDK1 and Cyclin B expression by Ru(II) complexes
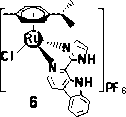
[0093] The effect of complexes on the expression of CDK1 and Cyclin B was analyzed by Western Blot. HeLa cells were cultured in a 60 mm tissue culture dish, when the cell density reached 70%, the complex was added at the specified concentration 3 and 6 , after incubation for 24 h, the protein samples were collected, and the prepared protein samples were denatured at 100 °C at high temperature, then SDS-PAGE gel electrophoresis, the required proteins were separated, transferred to the membrane, blocked, and incubated with primary antibodies (primary antibodies were CDK1 and Cyclin B and β-actin), secondary antibody incubation, protein detection. The experimental results show that the complex 3 and 6 It has a down-regulation effect on the expression of CDK1 and Cyclin B1, as shown in the figure below, as the concentration of the complex increases, the characteristic bands of CDK1 and Cycli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com