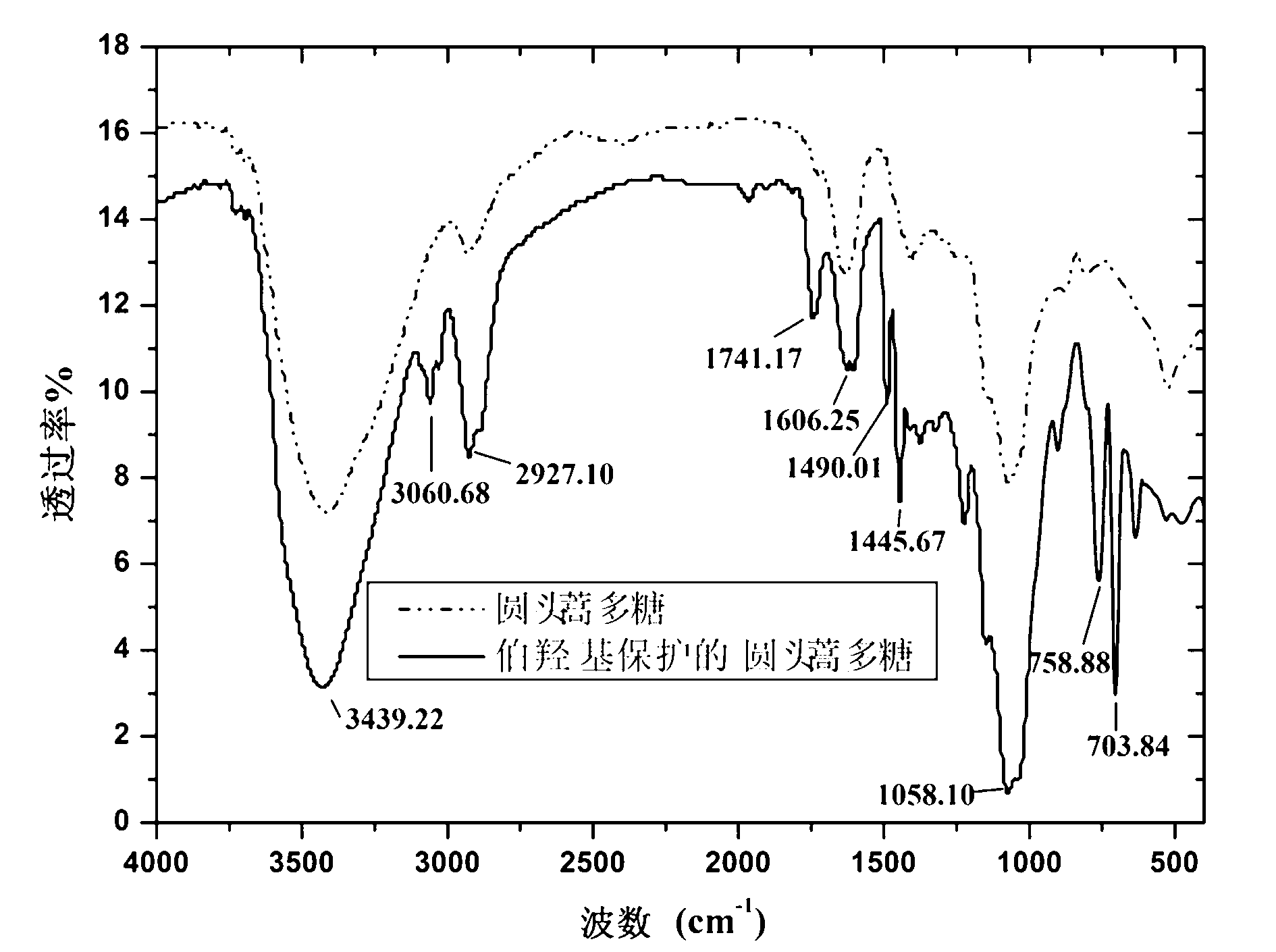
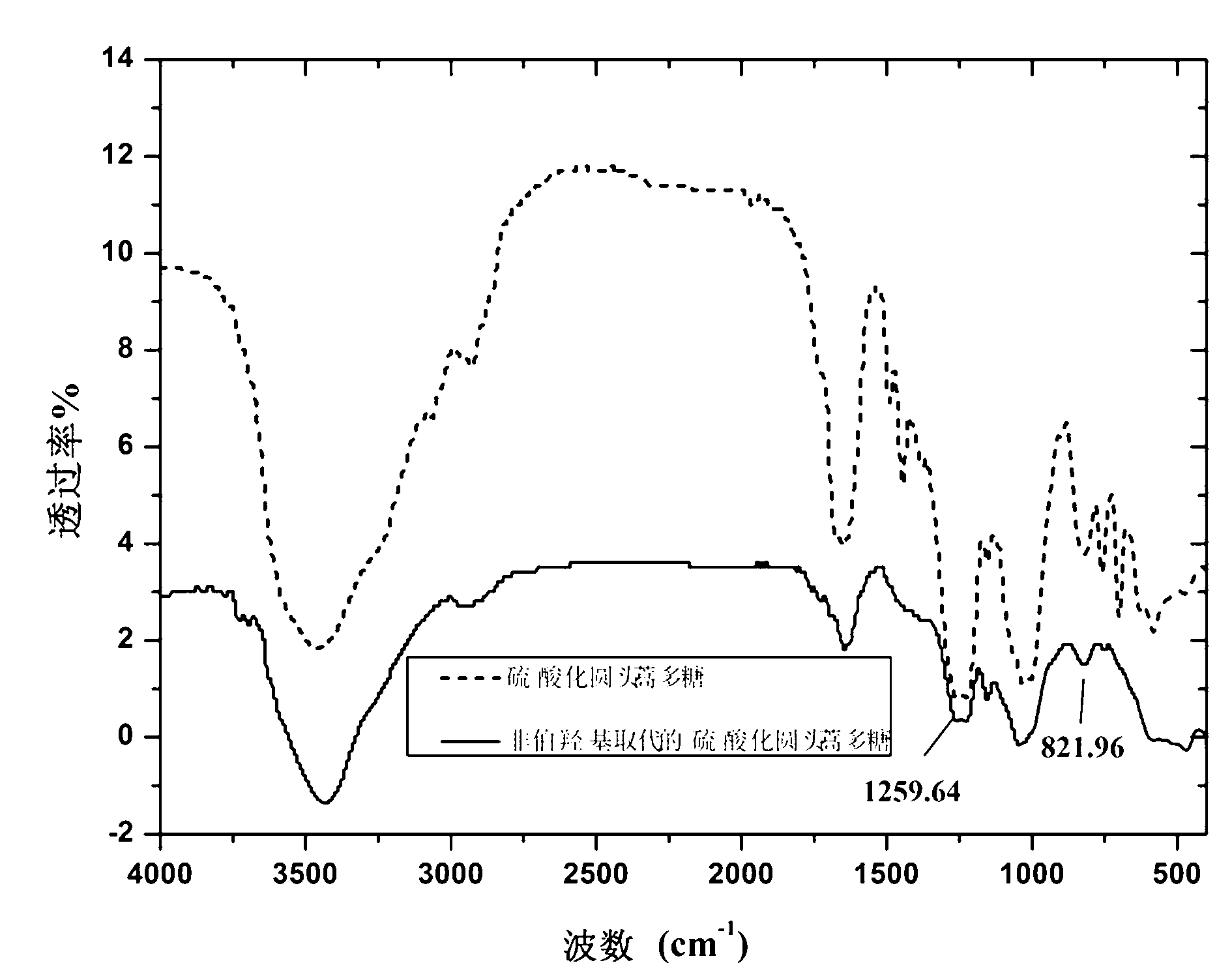
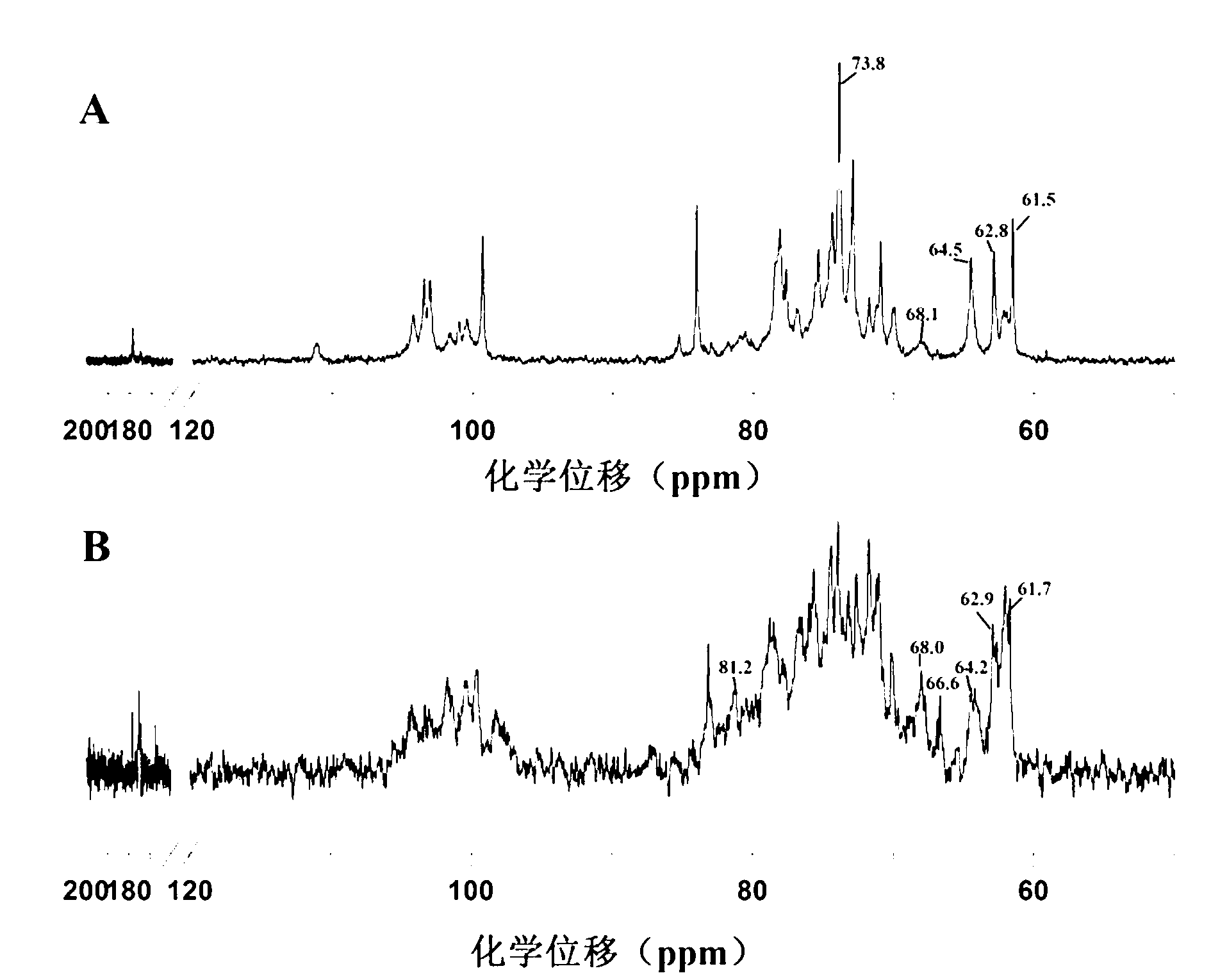
Synthesis method of sulfated polysaccharide substituted by non-primary hydroxyl group
A technology of sulfated polysaccharide and non-primary hydroxyl group is applied in the synthesis field of sulfated polysaccharide, which can solve the problems of strong steric hindrance effect, high difficulty and high molecular weight of polysaccharide, and achieves the effects of low cost, expanded application scope and suitable for popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of Artemisia arvensis polysaccharide solution: Weigh 0.45g Artemisia arvensis polysaccharide, add it into 15mL anhydrous formamide at room temperature, and stir for 5 hours at a speed of 1000 rpm on a magnetic stirrer to obtain a uniform disc. Artemisia polysaccharide solution;
[0047] (2) Protection of the primary hydroxyl group of polysaccharides from Artemisia arvensis: Weigh 2g of triphenylchloromethane and dissolve it in 5mL of pyridine at room temperature; then add it to the above solution of A. 2 protection, and react at 80°C for 9 hours; after the reaction is completed and cooled to room temperature, add 50mL of distilled water to wash, then wash with 100mL of absolute ethanol three times, filter at 0.07MPa, and dry the filter cake at 50°C for 10 hours; weigh and dry Filter cake 0.4g, add 10mL of anhydrous formamide at room temperature, stir for 3 hours at a speed of 800 rpm, and obtain the primary hydroxyl-protected artemisinin solution;
[00...
Embodiment 2
[0053] (1) Preparation of polysaccharide solution of Artemisia arvensis: Weigh 0.6g Artemisia annua polysaccharide, add it to 15mL anhydrous formamide at room temperature, and stir on a magnetic stirrer at a speed of 500 rpm for 6 hours to obtain a homogeneous arbor. Artemisinin solution;
[0054] (2) Protection of the primary hydroxyl group of polysaccharides from Artemisia argyifolia: Weigh 1g of triphenylchloromethane, dissolve it in 5mL pyridine at room temperature, and then add it to the polysaccharide solution of Artemisia aeruginosa, N 2 Protected, reacted at 70°C for 8 hours; after the reaction was completed and cooled to room temperature, add 40mL of distilled water to wash, then wash 3 times with 80mL of absolute ethanol, filter at 0.06MPa, and dry the filter cake at 60°C for 13 hours; weigh and dry Filter cake 0.3g, add 10mL anhydrous formamide at room temperature, stir with the rotating speed of 1200 rpm for 2 hours to get primary hydroxyl-protected artemisia polys...
Embodiment 3
[0060](1) Preparation of polysaccharide solution from Artemisia arvensis: Weigh 0.6g Artemisia annua polysaccharide, add it to 15mL of anhydrous formamide at room temperature, and stir for 4 hours on a magnetic stirrer at a speed of 1200 rpm to obtain a uniform arbor. Artemisinin solution;
[0061] (2) Protection of the primary hydroxyl group of polysaccharides from Artemisia argyifolia: Weigh 1g of triphenylchloromethane, dissolve it in 5mL pyridine at room temperature, and then add it to the polysaccharide solution of Artemisia aeruginosa, N 2 Protected, reacted at 75°C for 12 hours; after the reaction was completed and cooled to room temperature, add 60mL of distilled water to wash, then wash with 90mL of absolute ethanol three times, filter at 0.08MPa, and dry the filter cake at 40°C for 14 hours. Weigh 0.4 g of the dry filter cake, add 10 mL of anhydrous formamide at room temperature, and stir at a speed of 500 rpm for 4 hours to obtain a primary hydroxyl-protected artemi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap