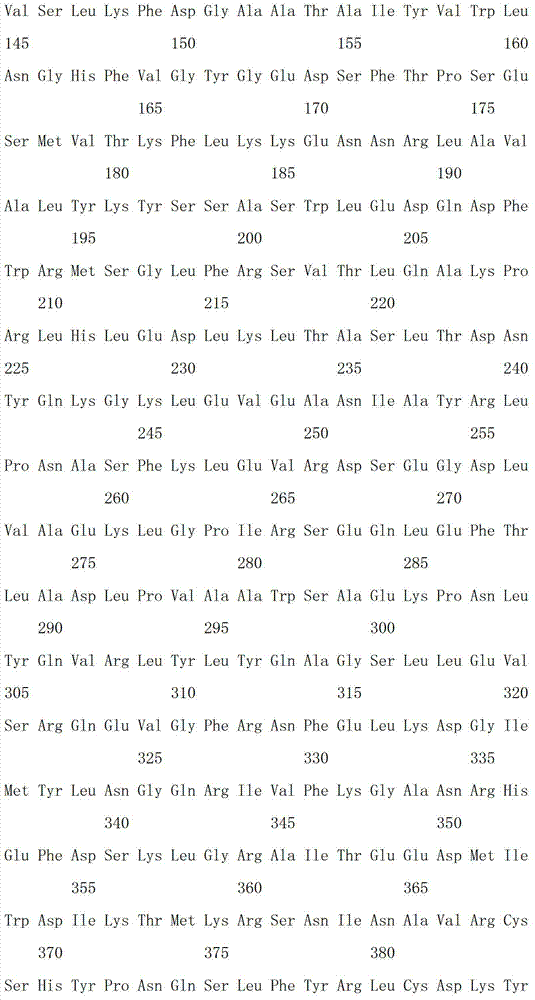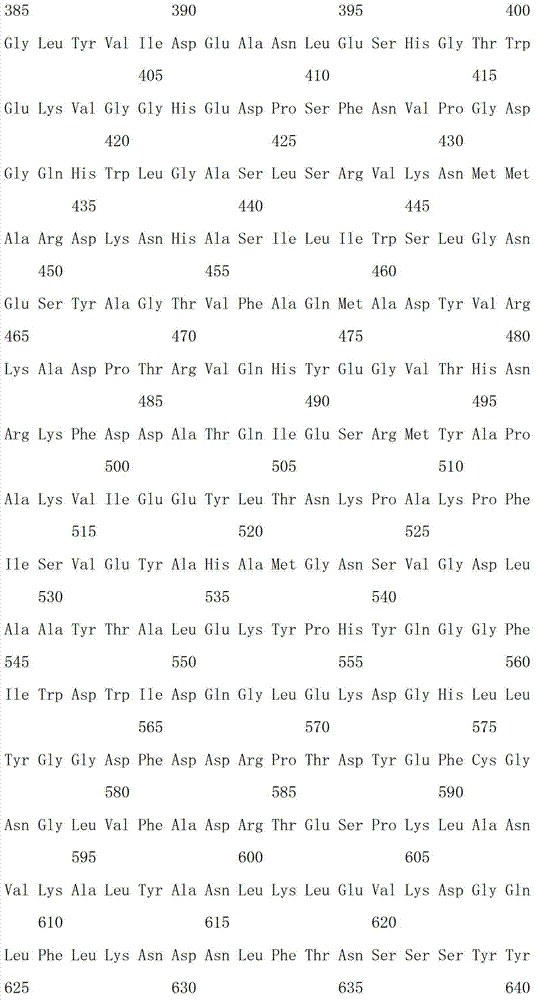Beta-galactosidase capable of glycosylating resveratrol
A technology of galactosidase and resveratrol is applied in the field of enzyme protein engineering, which can solve the problems of difficult glycosylation modification, low nucleophilicity of phenyl ring hydroxyl group, and difficulty in enzymatic modification of polyhydroxy phenols, etc. The effect of broadening the scope of transglycosylation receptors and having broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Acquisition of β-galactosidase gene capable of glycosylation of resveratrol and preparation of enzyme protein
[0032] Artificially synthesized β-galactosidase gene sequence with GenBank accession number EU734748.1 (encoded protein GenBank accession number is ACE06986.1), random mutation was performed by error-prone PCR:
[0033] Forward primer:
[0034] 5'-GC ATGAGCAATAAGTTAGTAAAAG-3' (containing NheI restriction site) reverse primer of SEQ ID NO.3:
[0035] 5'-GC TTATTTTAGTAAAAGGGGCTG-3 (with BglⅡ restriction site) SEQ ID NO.4
[0036] PCR reaction system (50 μL): template with a final concentration of 10 ng / μL, primers of 1 μM, 0.2 mM dATP, dGTP, 1 mM dTTP, dCTP, 5 mM MgCl 2 , 2.5U rTaq DNA polymerase, 1× PCR buffer.
[0037] PCR amplification conditions were: pre-denaturation at 95°C for 5 minutes; 30 cycles of reaction (denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 3 minutes); extension at 72°C for 10...
Embodiment 2
[0045] Example 2: Glycosylation of resveratrol by β-galactosidase
[0046]The final concentration of lactose was prepared with pH 7.0 and 50mM potassium phosphate buffer solution to be 0.2M, the final concentration of resveratrol was prepared with acetone to be 0.1M, the amount of enzyme added was 4μg / mL, and the reaction system was 50mL. After reacting at 45°C for 45 minutes, boil at 100°C for 5 minutes to terminate the reaction.
[0047] The boiled reaction solution was centrifuged at 12,000 rpm for 20 minutes, the supernatant was sucked, and the sample was spread on a preparative thin-layer chromatography plate (PLC Silica gel60F254, Merck). After the development, take a 1cm-wide strip plate every 10cm on the thin-layer chromatography plate for color development, and determine the position of the target sugar on the chromatography plate. Then scrape off the silica gel powder containing the target sugar in the non-colored area of the chromatographic plate, redissolve it i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com