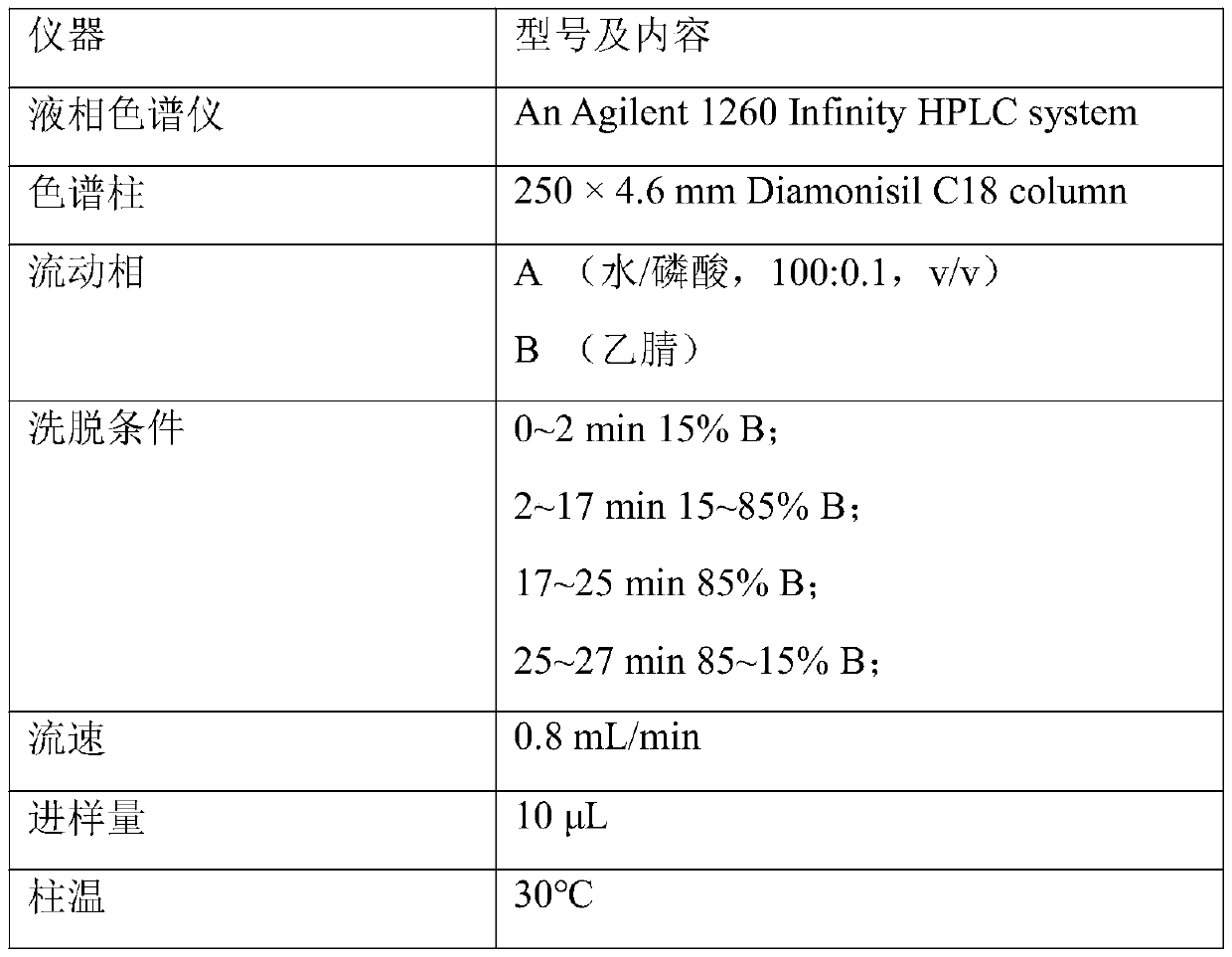
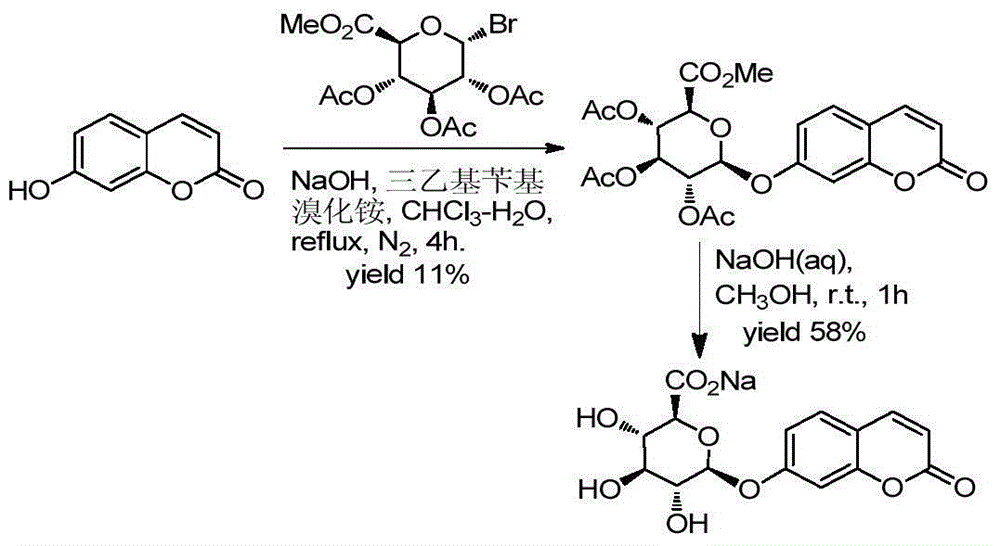
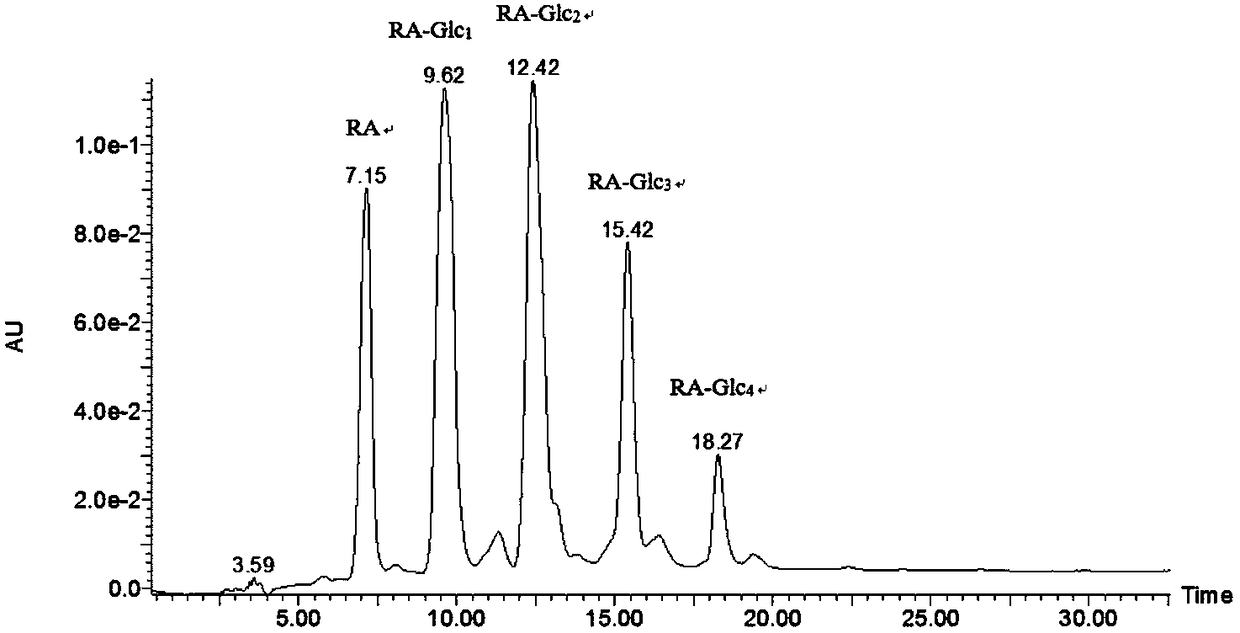
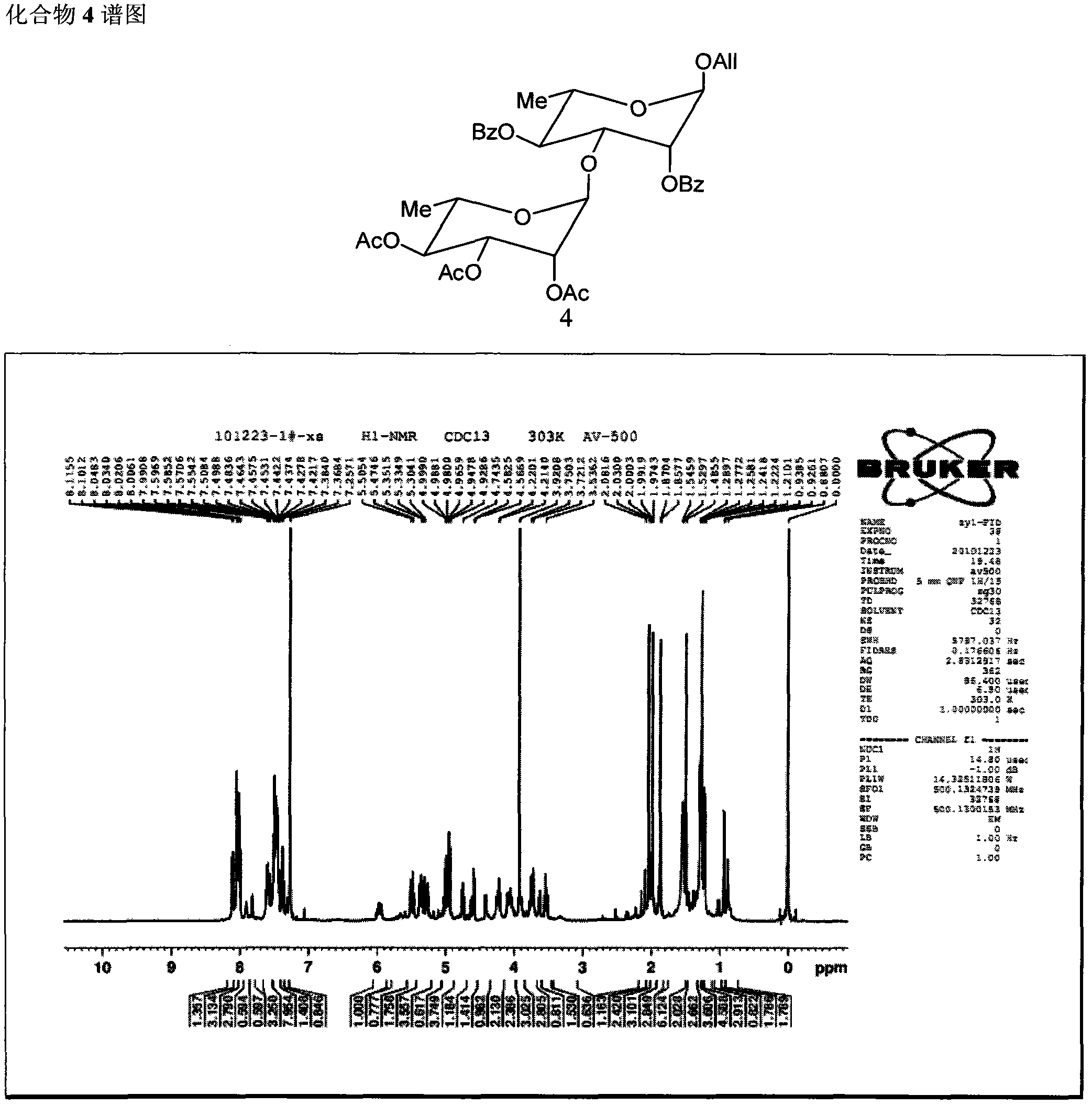
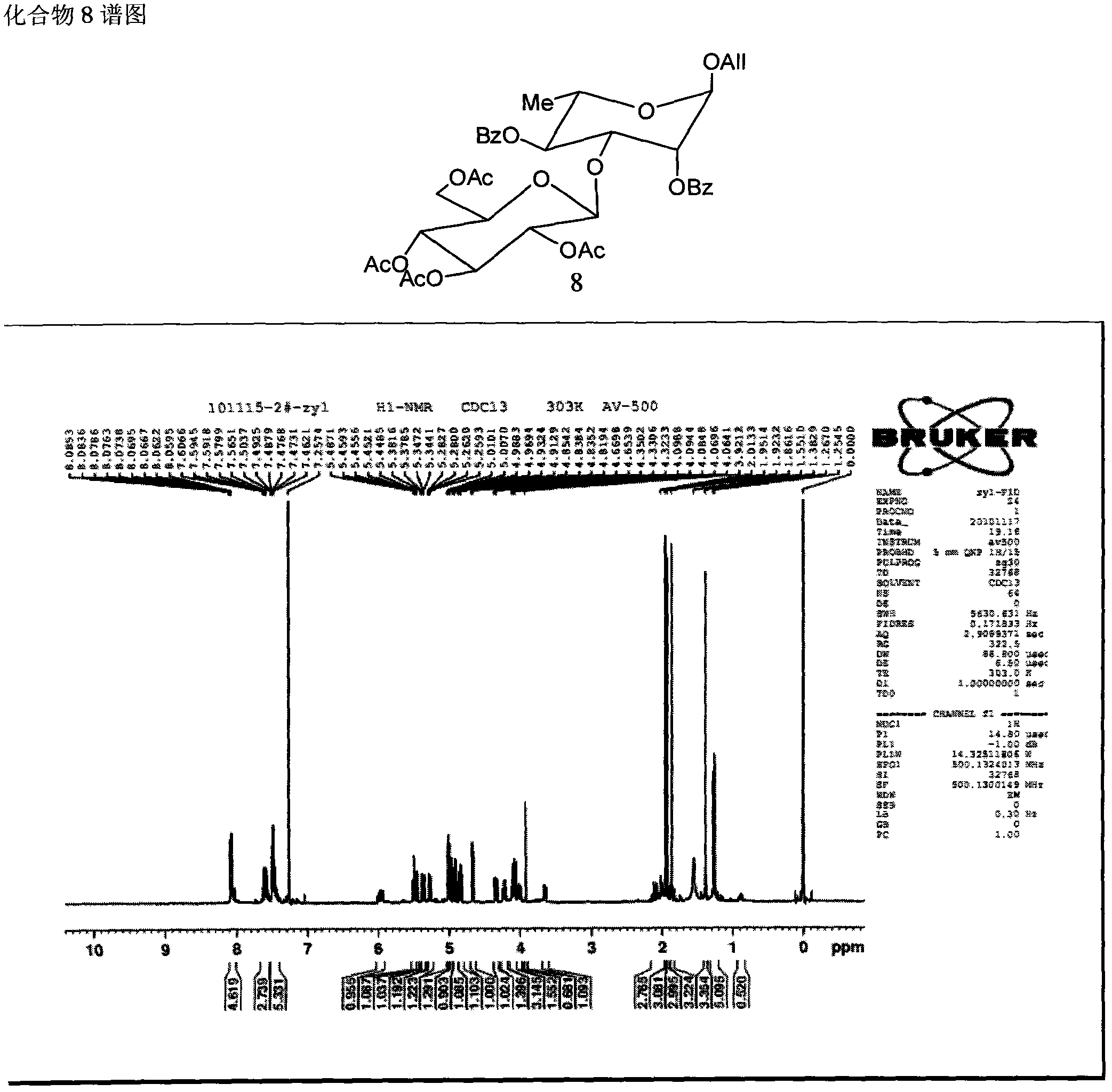
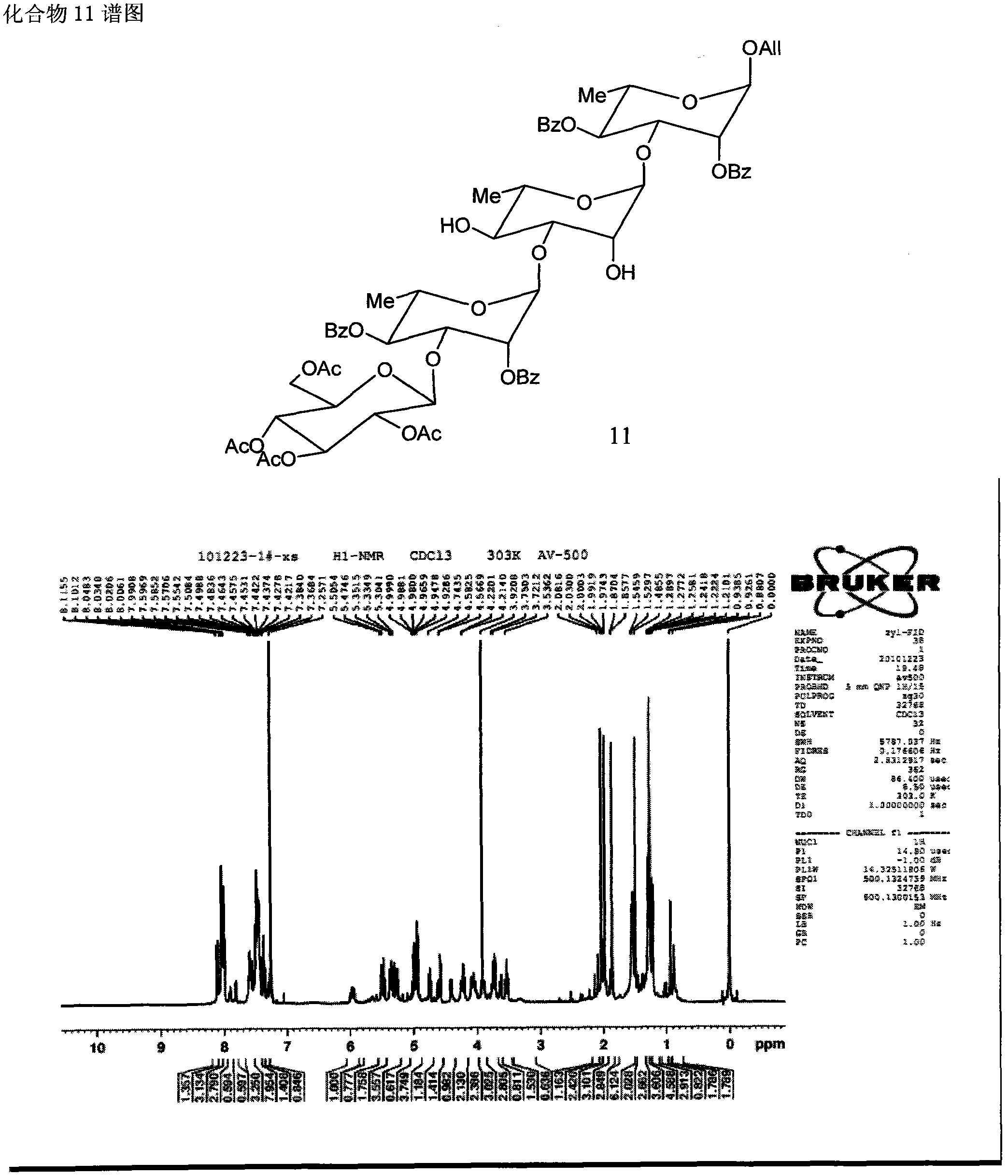
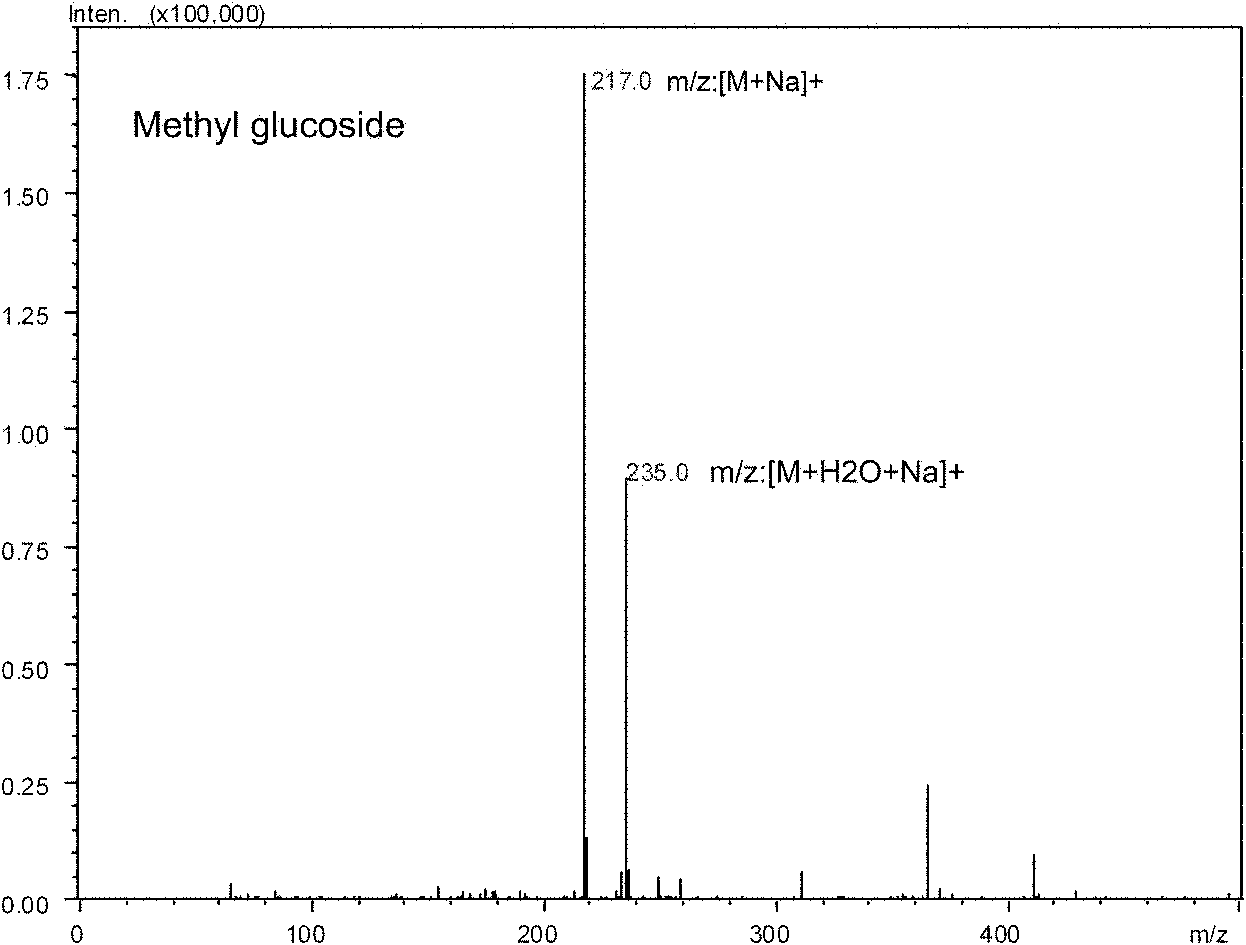
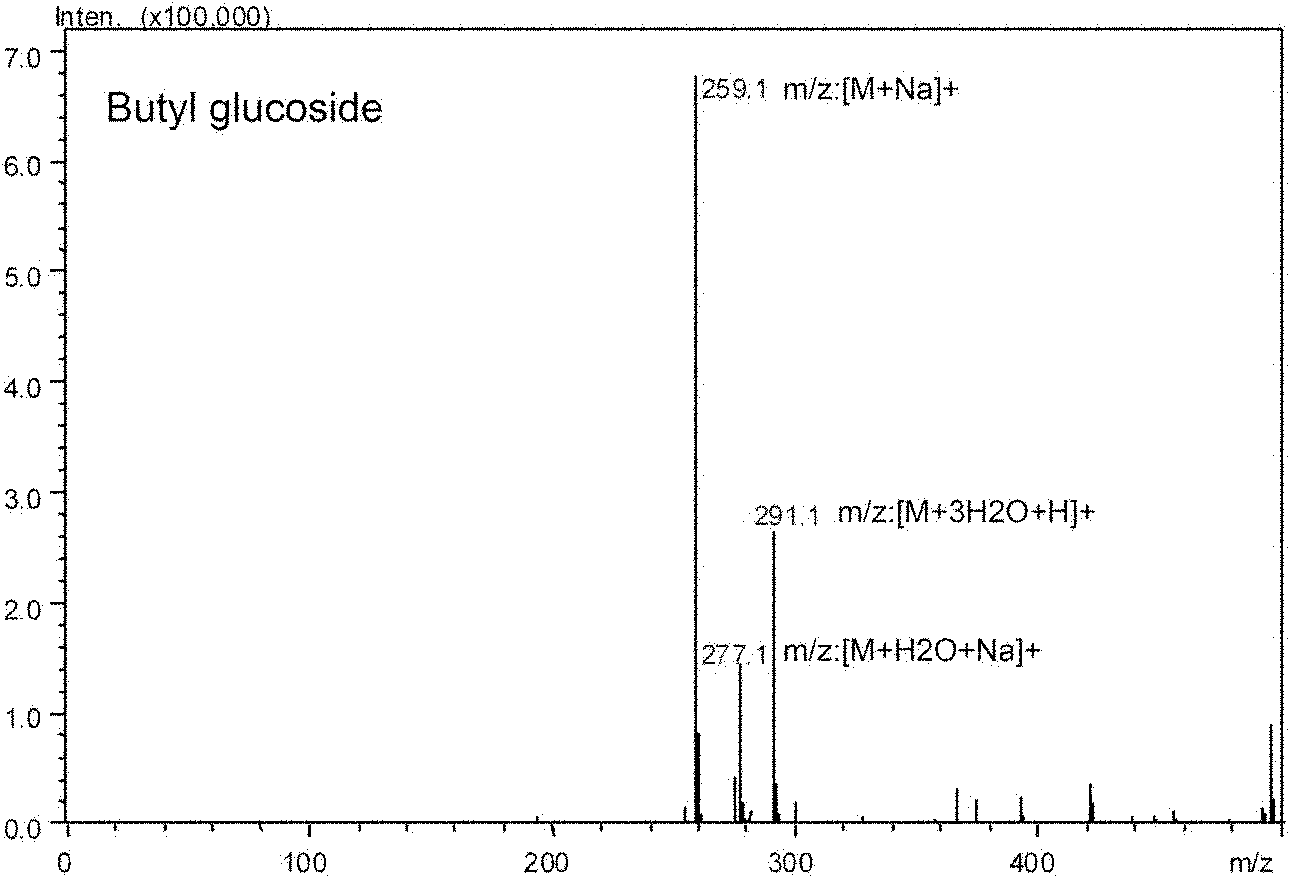
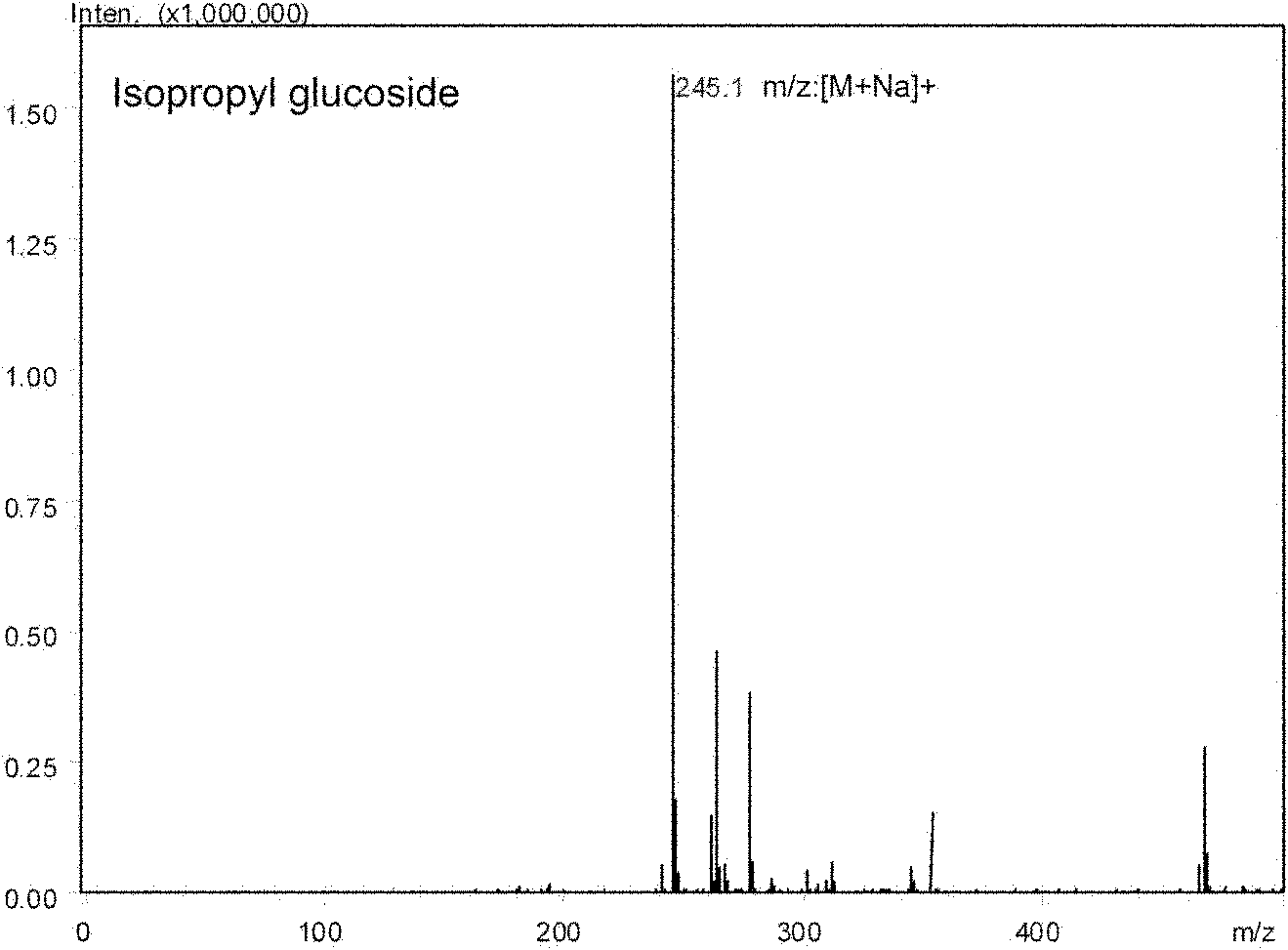
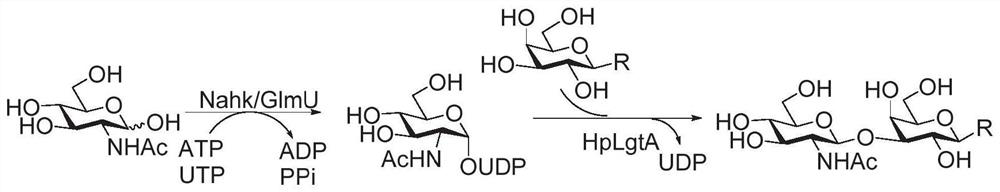
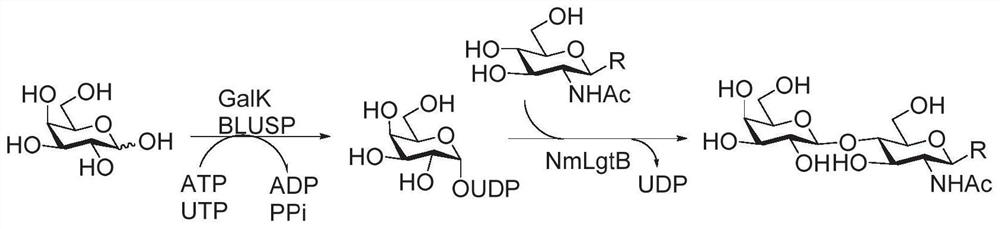
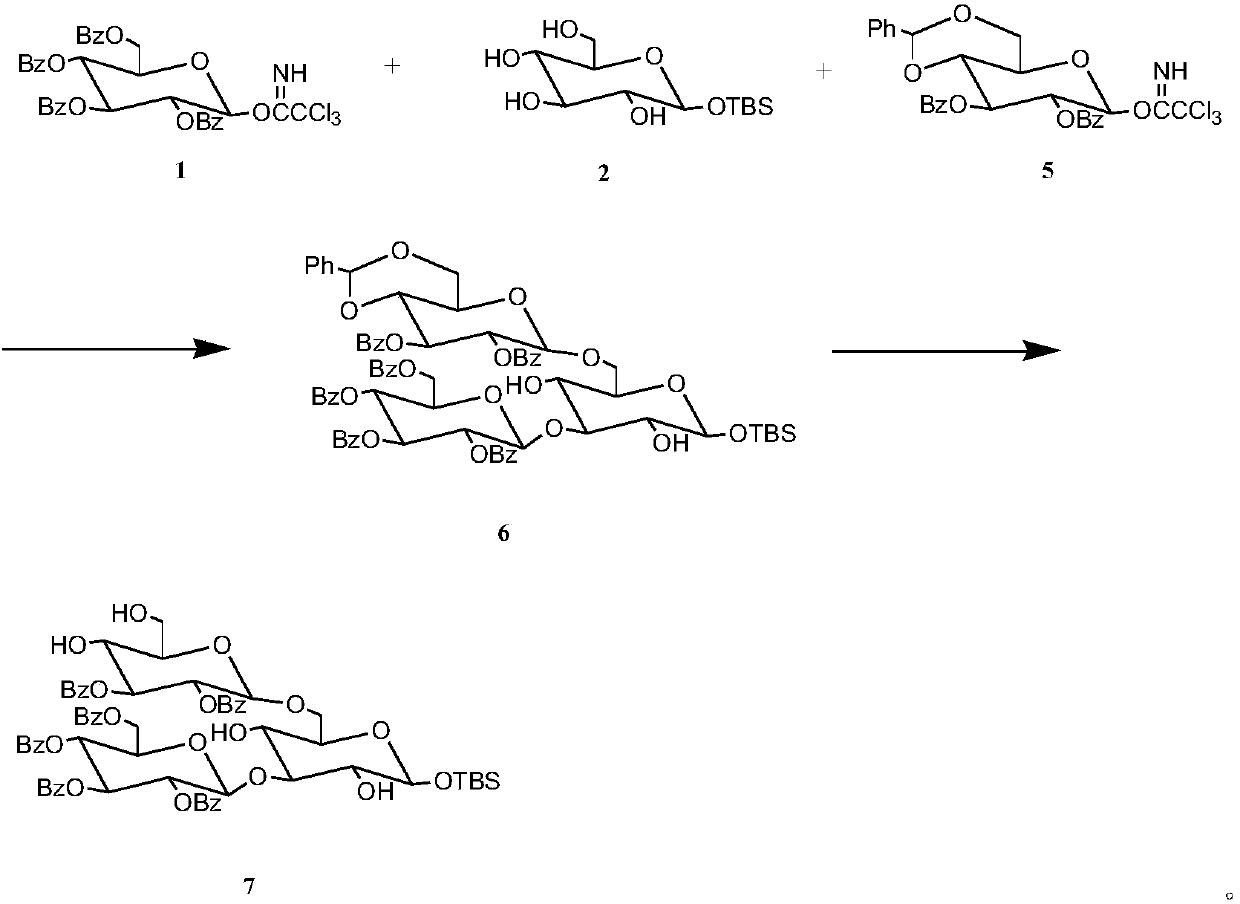
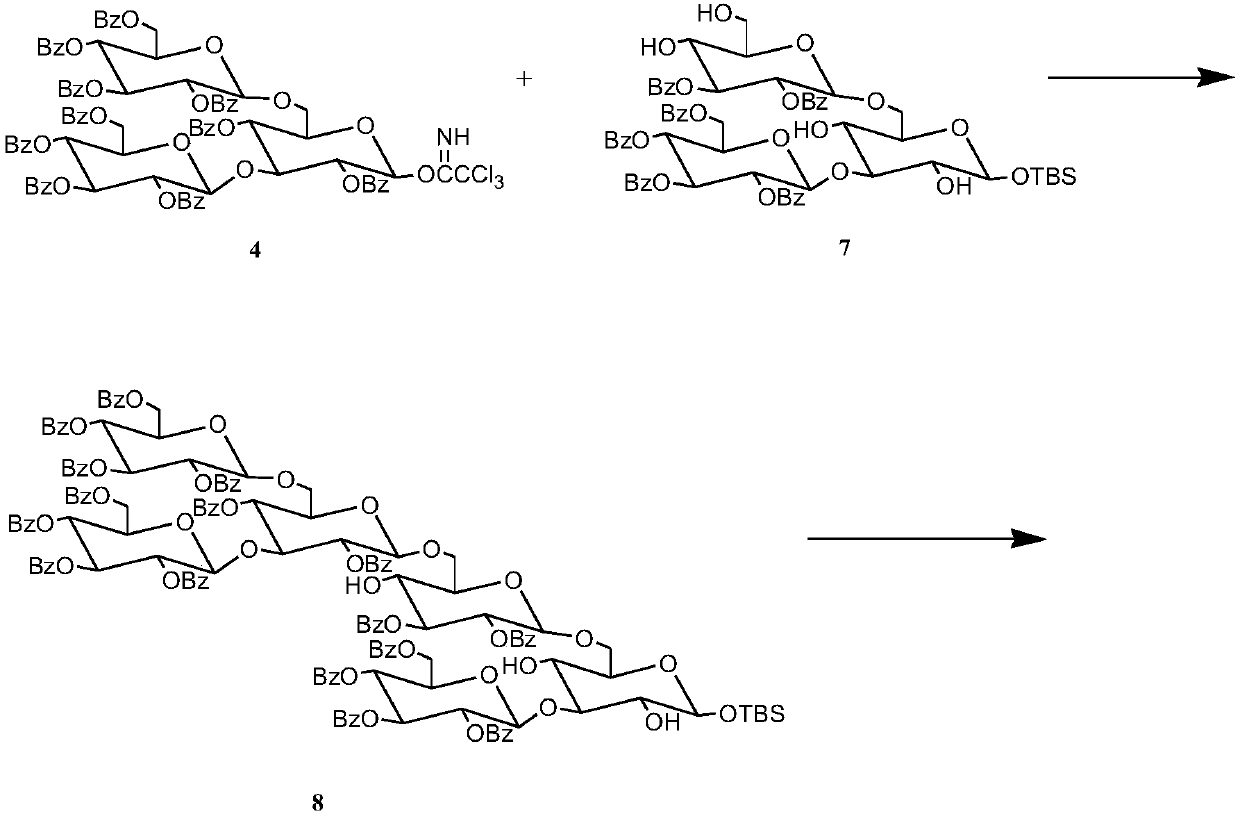
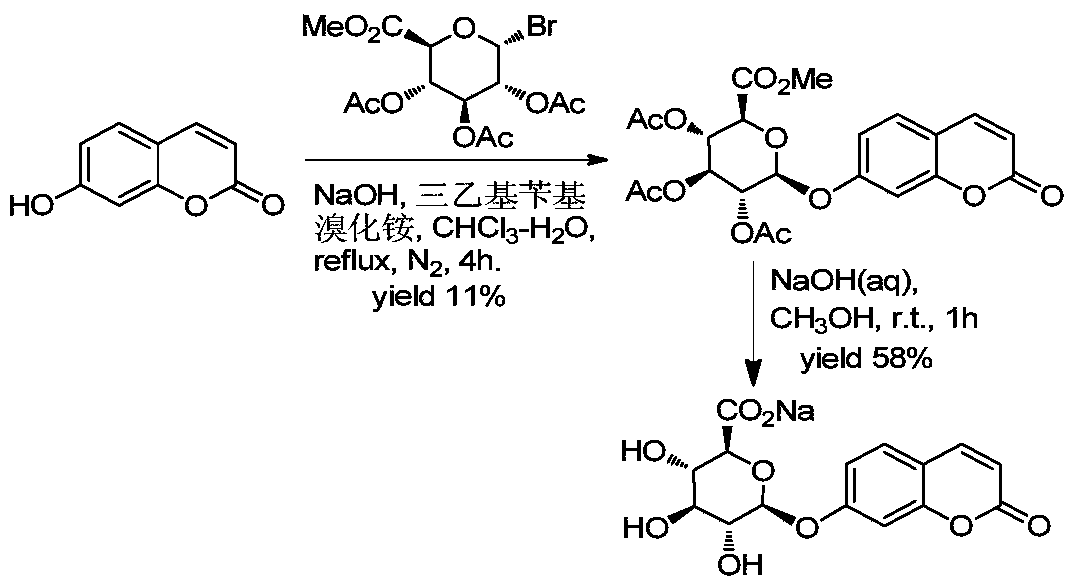
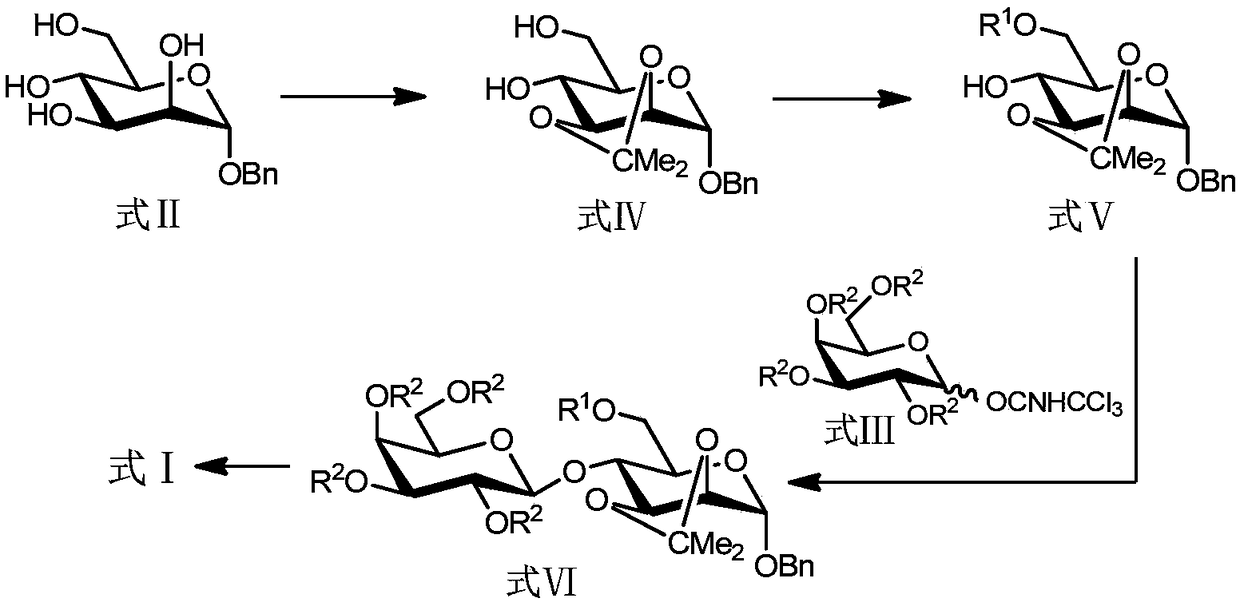
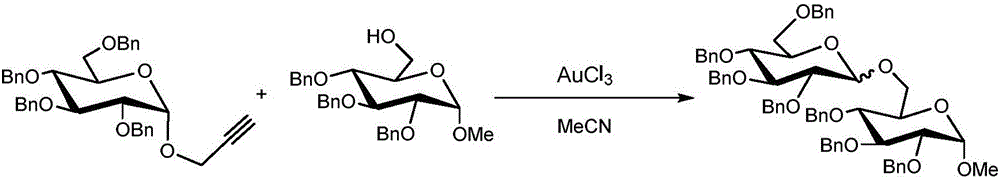
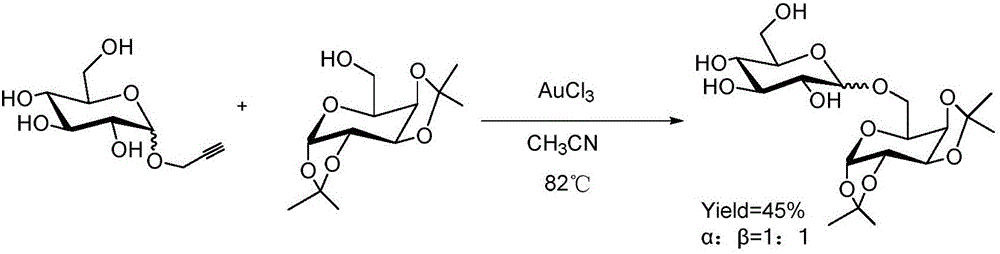
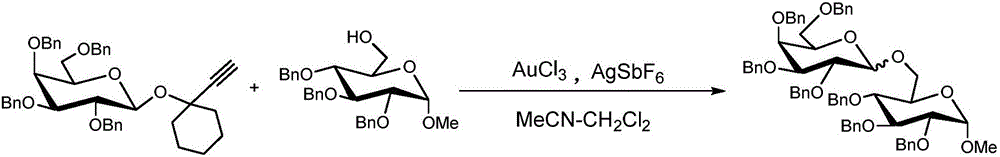
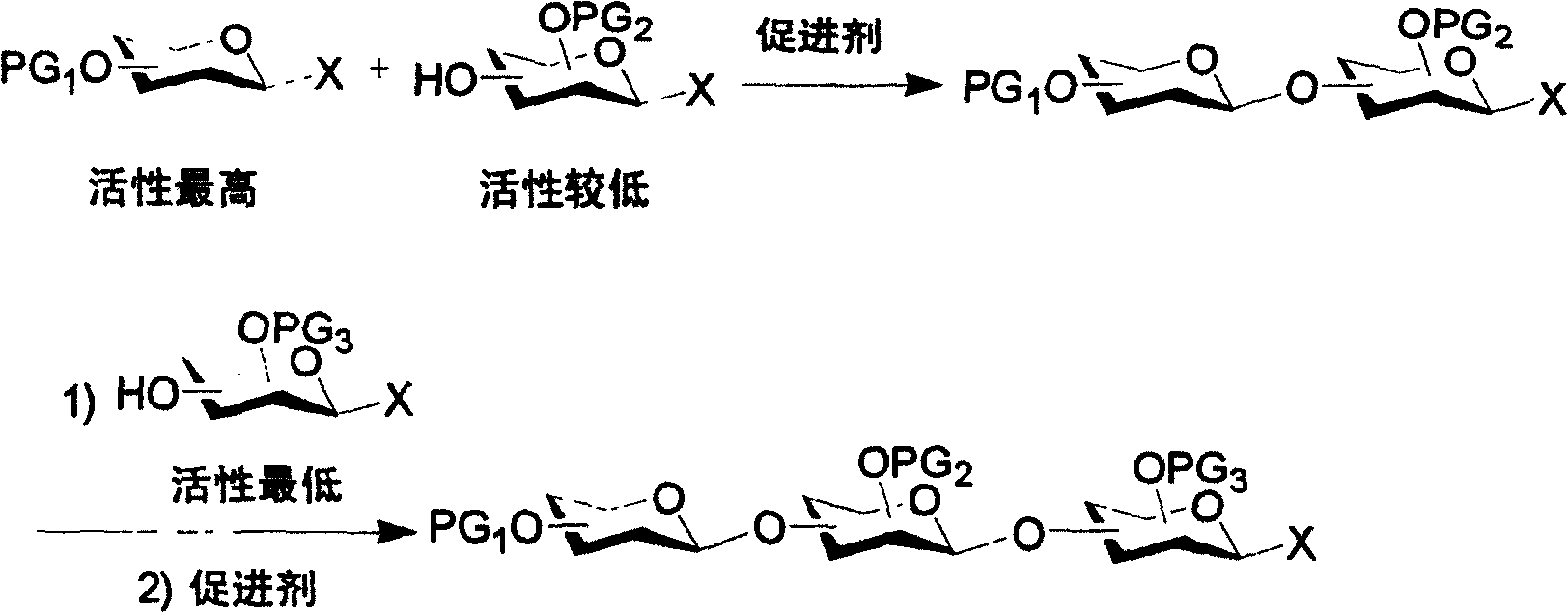
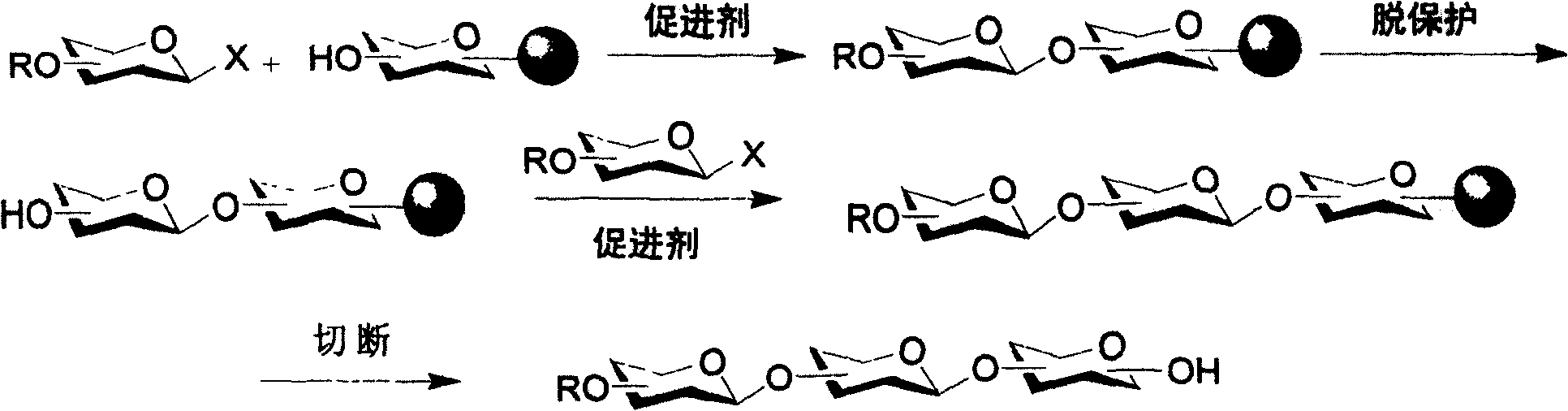
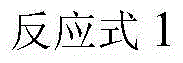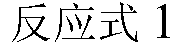Patents
Literature
45 results about "Glycosyl acceptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
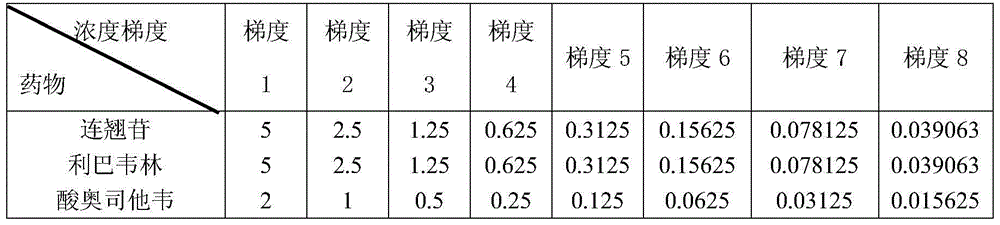
A glycosyl acceptor: is any suitable nucleophile-containing molecule that will react with a glycosyl donor to form a new glycosidic bond. By convention, the acceptor is the member of this pair which did not contain the resulting anomeric carbon of the new glycosidic bond. Since the nucleophilic atom of the acceptor is typically an oxygen atom, this can be remembered using the mnemonic of the acceptor is the alcohol. A glycosyl acceptor can be a mono- or oligosaccharide that contains an available nucleophile, such as an unprotected hydroxyl.
Method for improving water solubility of dye lignin by utilizing cyclodextrin glucosyltransferase transglycosylation reaction
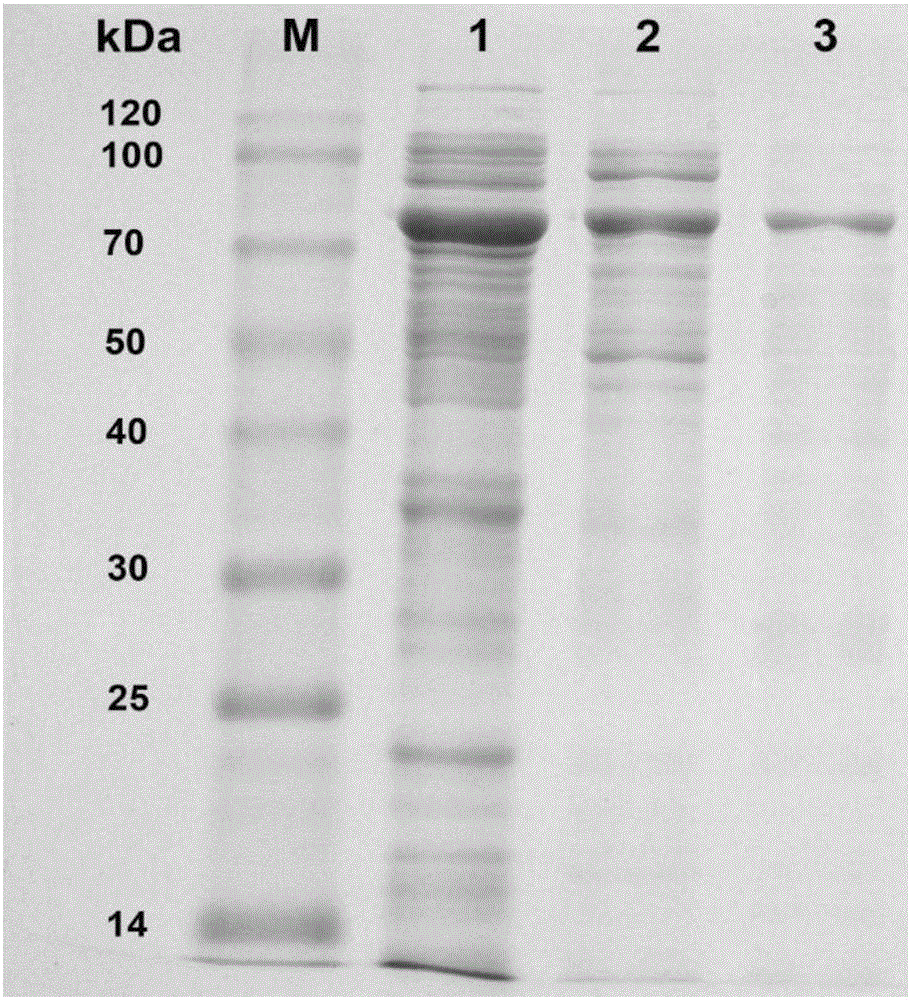
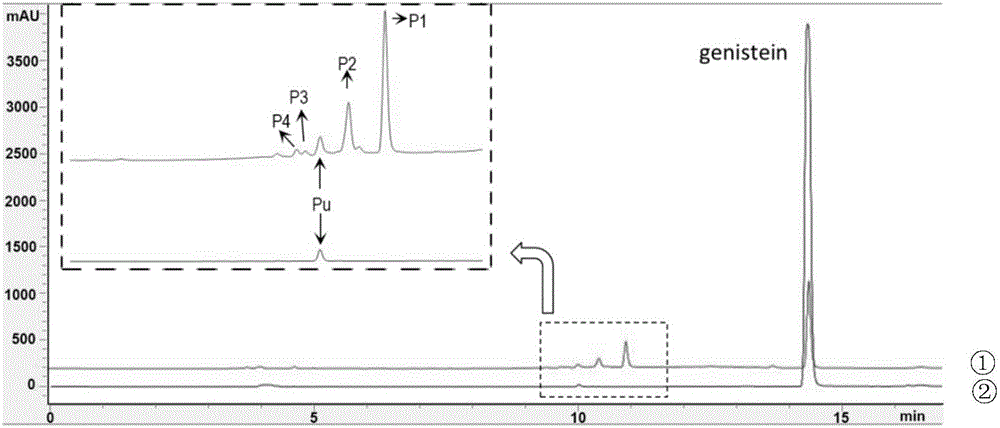
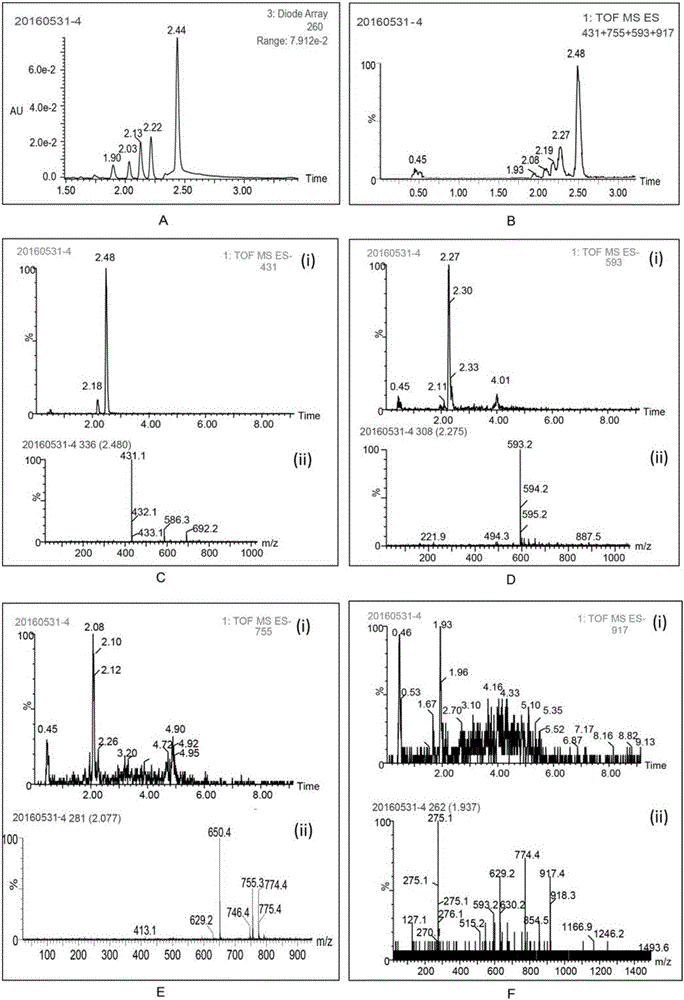
The invention discloses a method for improving the water solubility of dye lignin by utilizing cyclodextrin glucosyltransferase transglycosylation reaction, and belongs to the field of enzyme engineering. A gene (GenBank accession no.JX412224), which is derived from P.macerans strain JFB05-01 (CCTCC NO:M208063) and optimized by a cyclodextrin glucosyltransferase codon, is connected to pET20b(+) plasmid and is transferred to an E.coli BL21(DE3) cell to establish gene engineering bacteria for producing the cyclodextrin glucosyltransferase. The pure cyclodextrin glucosyltransferase, which is obtained through fermentation enzyme production and purification, is applied to catalytic transglycosylation reaction, alpha-cyclodextrin serves as a glycosyl donor, the dye lignin serves as glycosyl receptor, and at least four dye lignin glycosylated derivatives (Glc)n-genistein (n is equal to 1, 2, 3 and 4) are finally obtained, wherein Glc-genistein and (Glc)2-genistein are main products, in particular the water solubility of the (Glc)2-genistein is greatly improved compared with that of the dye lignin.
Owner:JIANGNAN UNIV
Cyclodextrin glycosyltransferase mutant and application thereof
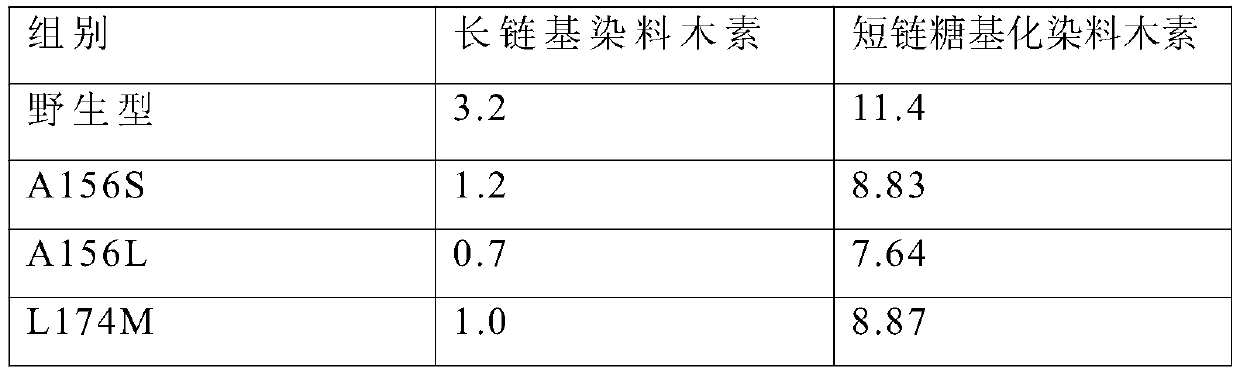
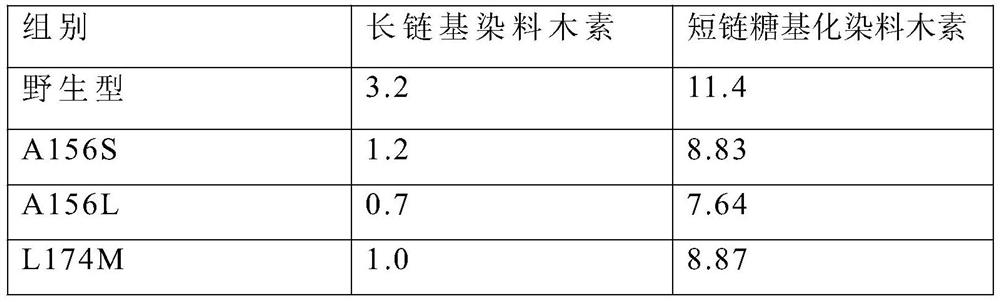
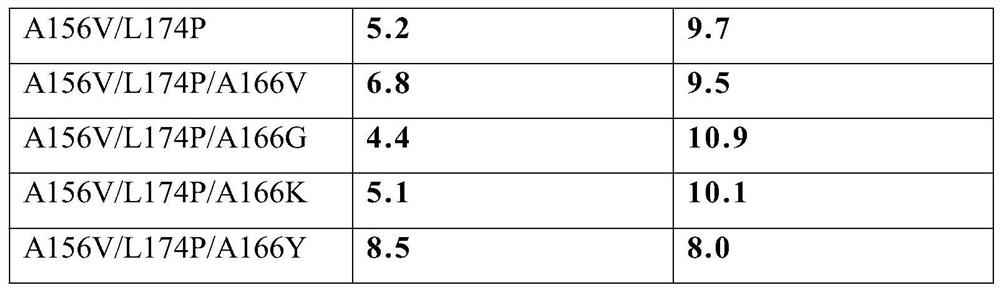
The invention discloses cyclodextrin glucosyltransferase mutants and application thereof, and belongs to the technical field of enzyme engineering and microbial engineering. The CGTase mutants have high specificity to long-chain glycosylated genistein products. The yield of long-chain glycosylated genistein prepared from CGTase mutants A156V / L174P, A156V / L174P / A166Y, A156V / L174P / A166V, A156V / L174P / A166G and A156V / L174P / A166K by using maltodextrin as a glycosyl donor and genistein as a glycosyl receptor is increased by 62.5%, 165%, 112.5%, 112.5% and 59.4% respectively, compared with the yieldof long-chain glycosylated genistein prepared from wild type cyclodextrin glucosyltransferase by taking maltodextrin as a glycosyl donor and genistein as a glycosyl receptor.
Owner:JIANGNAN UNIV
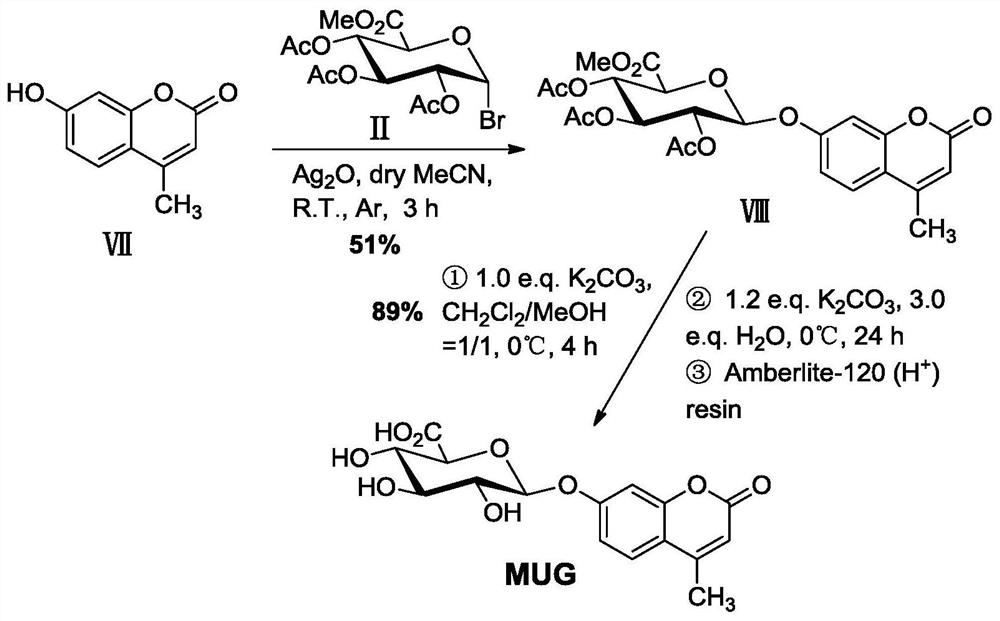
Method for synthesizing various glucosides on basis of 4-methylumbelliferone
The invention discloses a method for synthesizing various glucosides on a basis of 4-methylumbelliferone. According to the invention, a glycosyl donor peracetyl saccharide and a glycosyl acceptor 4-methylumbelliferone are subjected to a glycosylation reaction under room temperature or under heating with dichloromethane or 1,2-dichloroethane as a solvent and with the combined effect of Lewis acid boron trifluoride ethyl ether and organic alkali triethylamine or pyridine; and protecting groups are removed, such that various glucosides based on 4-methylumbelliferone can be obtained. The glucosides include 4-methylumbelliferone-beta-D-glucopyranosiduronide, 4-methylumbelliferone-beta-D-glucopyranoside, 4-methylumbelliferone-beta-D-xylopyranoside, 4-methylumbelliferone-beta-D-ribofuranoside, 4-methylumbelliferone-alpha-D-galactopyranoside, and 4-methylumbelliferone-alpha-D-mannopyranoside. The method is simple, and can produce a beta or alpha single-configuration target. A glycosylation reaction yield can reach 17-93%.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
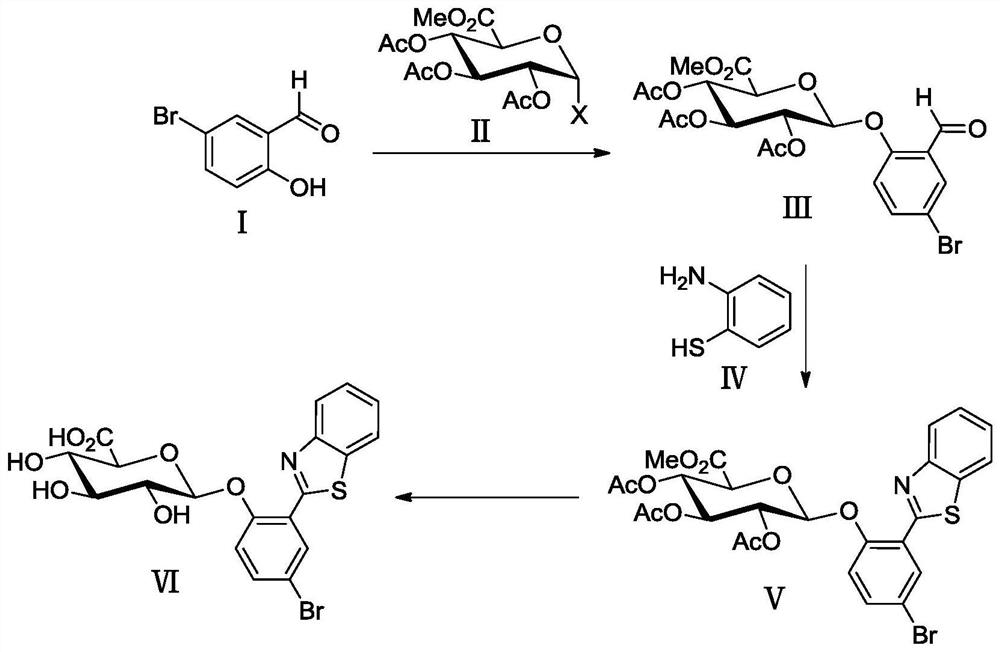
Synthesis method of glucoside based on indoxyl derivative and 2-(benzothiazol-2'-yl)phenol derivative
ActiveCN106432369AEasy to operateHigh yieldSugar derivativesFluorescence/phosphorescenceMicroorganismIndoxyl
The invention discloses a synthesis method of glucoside based on an indoxyl derivative and a 2-(benzothiazol-2'-yl)phenol derivative. The general formula of a synthetic equation of the synthesis method is as shown in the description; the Ar-OH represents aromatic phenol or a ketonic compound thereof, and is used as a glycosyl receptor in a glycosylation reaction; the G-X represents halogenated sugar, and is used as a glycosyl donor in the glycosylation reaction. According to the synthesis method of the glucoside based on the indoxyl derivative and the 2-(benzothiazol-2'-yl)phenol derivative, a 1-acetylindolin-3-one derivative is selected and used as an intermediate; a novel synthesis method is researched and developed to prepare the glucoside based on the indoxyl derivative; a novel glycosidase fluorescence probe based on a BTP ((2-benzothiazol-2'-yl)phenyl) derivative is synthesized by applying the method; meanwhile, the application effects of the glucoside based on the indoxyl derivative and the glycosidase fluorescence probe are investigated. The glucoside based on the indoxyl derivative and the 2-(benzothiazol-2'-yl)phenol derivative can be used as glycosidase substrates, and is used for the positioning analytic detection and screening on the corresponding glycosidase, a microorganism using the corresponding glycosidase as a characteristic target, and the like.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
Method for preparing glucose-based stevioside by enzymatic variable temperature and high-flux
The invention discloses a method for preparing glucose-based stevioside by enzymatic variable temperature and high-flux, and belongs to the technical field of sweetener biosynthesis. According to themethod for preparing the glucose-based stevioside by the enzymatic variable temperature and high-flux, cyclodextrin glucosyltransferase derived from geobacillus sp. is used as a catalyst, stevioside is used as a glycosyl acceptor, dextrin or oligosaccharide is used as a glycosyl donor, and a calcium barium ion salt bridge is used as a main stabilizer, and glycerol is combined with to adjust the conformation and binding domain opening degree of enzyme, the variable temperature utilizes the transesterification and hydrolysis activity of amylase by stages to prepare the glucose-based stevioside by enzymatic variable temperature and high-flux. The technology disclosed by the invention can improve the utilization rate of the enzyme, and obtain the glucose-based stevioside with excellent sweetness and good taste.
Owner:JIANGNAN UNIV
Method for preparing allolactose
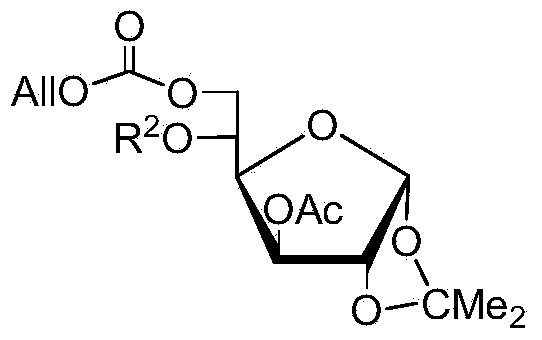
InactiveCN103665054AHigh yieldHigh purityEsterified saccharide compoundsSugar derivativesFuranGlycosyl
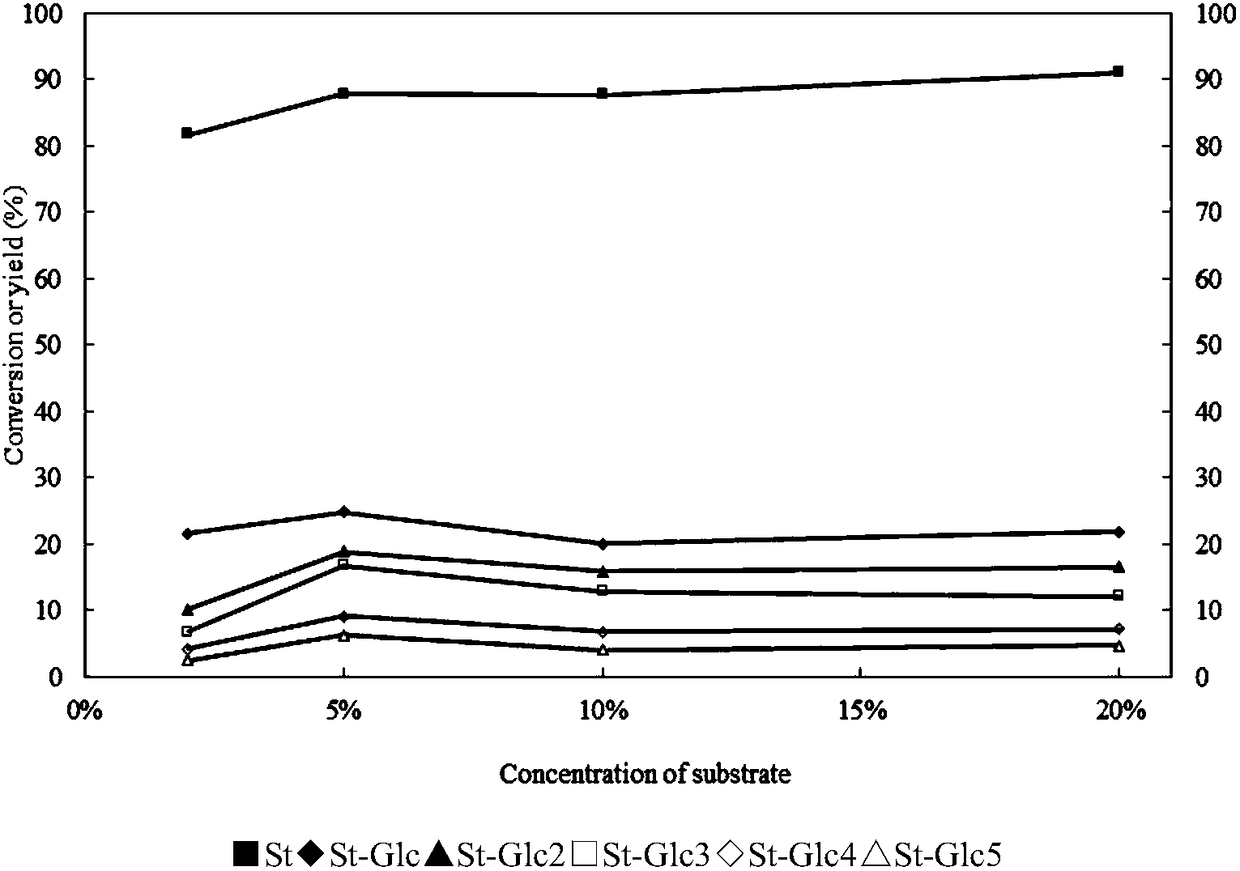
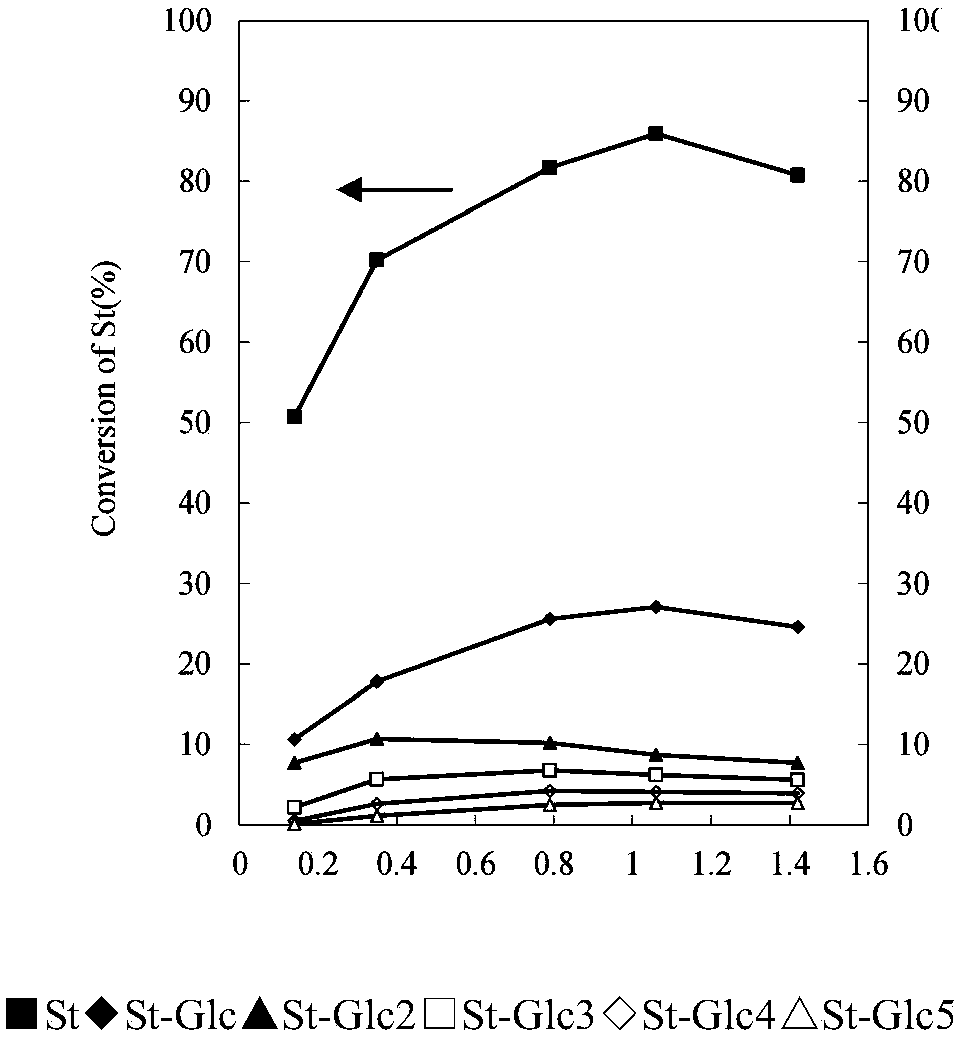
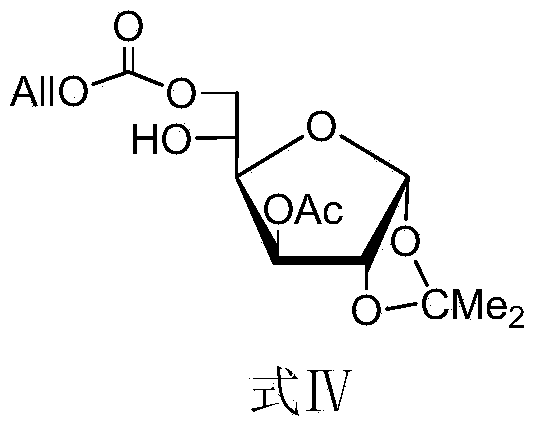
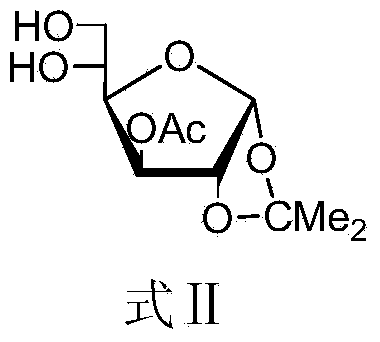
The invention discloses a method for preparing allolactose. According to the method, 1,2-O-(1-methyl ethylidene)-3-O-acetyl-alpha-D-furan glucose is used as an initial raw material, a glycosyl receptor 1,2-O-(1-methyl ethylidene)-3-O-acetyl-5-O-benzoyl-alpha-D-furan glucose midbody is obtained through three steps of reaction, the yields and the purities of each step of reaction are all high (the yield is respectively 97%, 100% and 95%, and the purities are respectively 99.5%, 99.2% and 99.8%). Therefore, midbodies IV and V can be applied to next step of reaction without being purified in large-scale preparation, a compound formula VII is obtained by coupling a midbody formual VI and a midbody formula V, the reaction condition is obtained through optimization, the yield is high (80%), the stereoselectivity is good (no isomer is detected), purification can be easily achieved, the total yield that a disaccharide formula VII is prepared from a compound formula II through four steps of reaction is 74%, the purity is 99.8%, and the purity of a final product of the invention is high, that is, the purity is not less than 98%.
Owner:CHINA AGRI UNIV
Method for determining activity of O-linked N-acetylglucosamine transferase and application of method
InactiveCN111926056ASolve the problem of low catalytic activityEasy to operateMicrobiological testing/measurementBiological material analysisReceptorBovine serum albumin
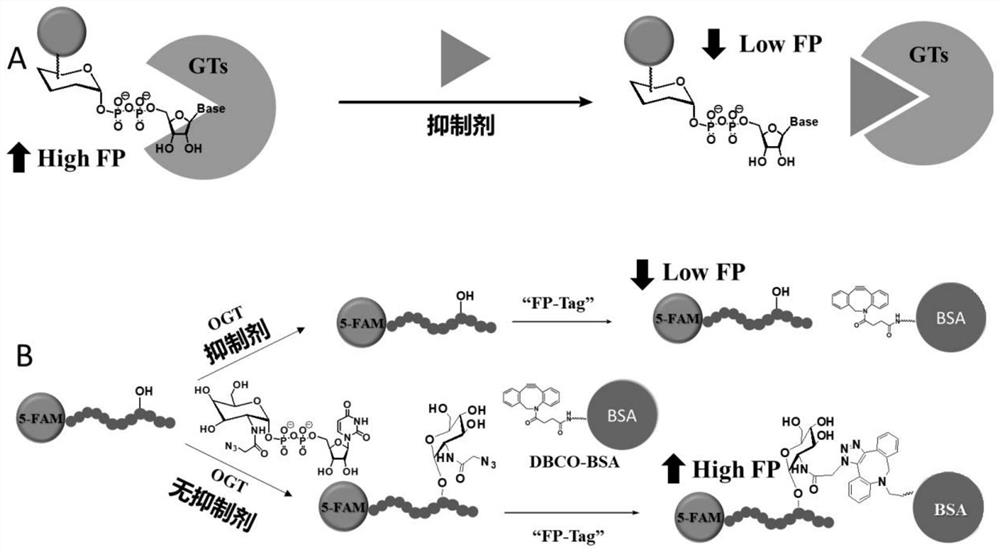
The invention relates to the technical field of biological analysis and detection, in particular to a method for determining the activity of O-linked N-acetylglucosamine transferase and application ofthe method. The method comprises the following steps: firstly, modifying a glycosyl receptor of OGT by utilizing a fluorescent group, transferring azide-modified glycosyl to polypeptide through an enzyme reaction catalyzed by OGT, and then connecting the glycosyl receptor to macromolecular bovine serum albumin modified by dibenzocyclooctyne through a click reaction. The property that bovine serumalbumin is a macromolecule is utilized, and the bovine serum albumin is used as an 'FP tag 'capable of causing polarization signal enhancement, so that the enzyme activity of OGT is visually displayed. According to the invention, the problem of low catalytic activity of OGT on a complex modified substrate is solved, meanwhile, the correlation between the enzyme activity and the fluorescence polarization signal size is realized, and the method can be used for determining the enzyme activity of OGT, and can also be used for high-throughput screening of an OGT inhibitor and determination of theinhibitory activity of the OGT inhibitor.
Owner:SUN YAT SEN UNIV
Method for synthesizing beta-D glucose(1->3)alpha-L rhamnose(1-3)alpha-L rhamnose(1-3)alpha-L rhamnose
The invention provides a method for synthesizing beta-D glucose(1->3)alpha-L rhamnose(1->3)alpha-L rhamnose(1->3)alpha-L rhamnose. The method comprises the following steps of: obtaining 1,3-alpha-connection disaccharides and 1,3-beta-connection disaccharides by trichloroacetimidate of acyl rhamnose and acyl glucose serving as a glycosyl donor and 1-O-allyl rhamnose serving as a glycosyl acceptor; coupling the 1,3-alpha-connection disaccharides serving as a glycosyl acceptor and the 1,3-beta-connection disaccharides serving as a glycosyl donor to obtain tetrasaccharides; and removing the protection to obtain the beta-D glucose(1->3)alpha-L rhamnose(1->3)alpha-L rhamnose(1->3)alpha-L rhamnose.
Owner:NANJING UNIV OF TECH
Microbes for alkyl glycoside synthesis and application thereof
The invention relates to microbes having a high-efficiency transglycosylation activity and application thereof. The microbes are Arthrobacter sp.DL001. Strains or broth of Arthrobacter sp.DL001 can realize high-efficiency transglycosylation to glycosyl acceptors in the presence of glycosyl donors and thus a novel effective method for preparing carbohydrates and glycosides by biotransformation reactions is provided. The novel effective method can realize high-efficiency, cheap and eco-friendly preparation of carbohydrates and glycosides and is an effective approach of industrial production of carbohydrates and glycosides.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
Fluorine label and preparation method thereof, and method for synthesizing oligosaccharide chain by adopting assisted enzymic method
ActiveCN112940058AHigh modular assembly efficiencyEfficient preparationSugar derivativesOligosaccharidesReceptorCombinatorial chemistry
The invention relates to a fluorine label and a preparation method thereof, and a method for synthesizing an oligosaccharide chain by adopting an assisted enzyme method. The structural formula of the fluorine label is G-R(I) as shown in the formula I. G represents monosaccharide or oligosaccharide; and R is one of the following formulas II and III, wherein R1 is one of C6H13 and C8H17. When G represents lactose and R represents a C8F17 difluoro chain, the formula II is shown in the specification. The fluorine label has the advantages of being easy to remove and recycle. The glycosyl receptor is used for synthesizing various different oligosaccharide chains, so that the oligosaccharide chain with a determined structure is quickly and efficiently synthesized.
Owner:SHANDONG UNIV
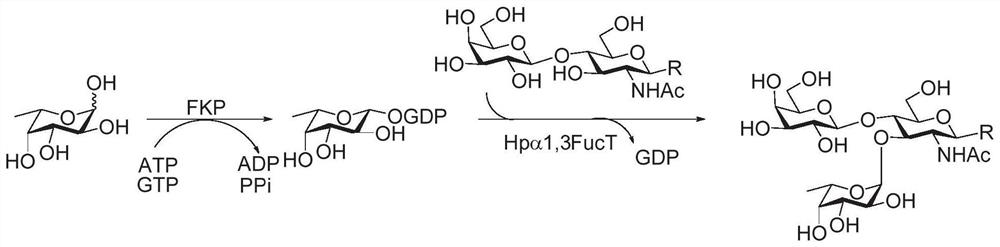
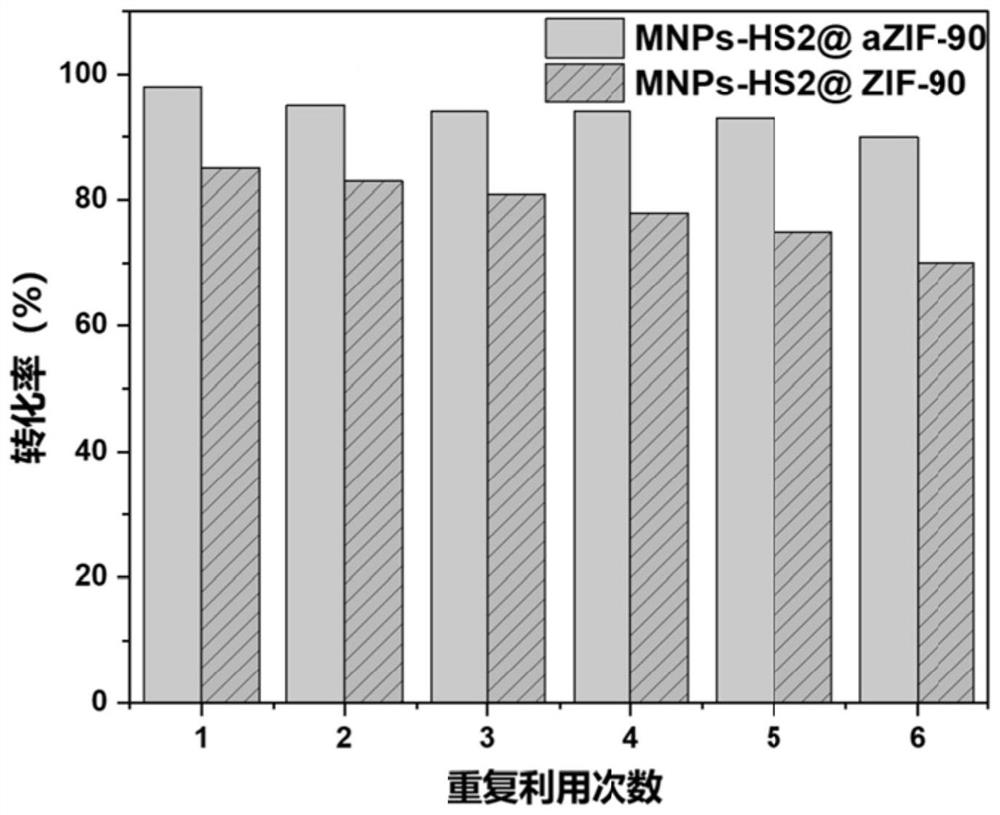
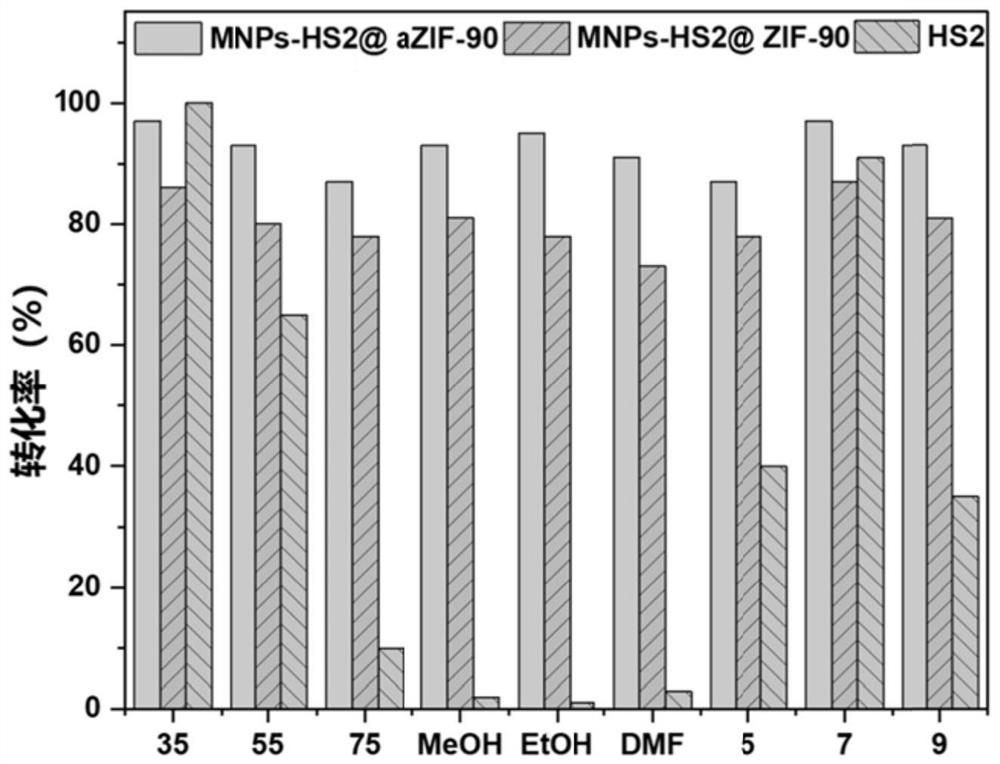
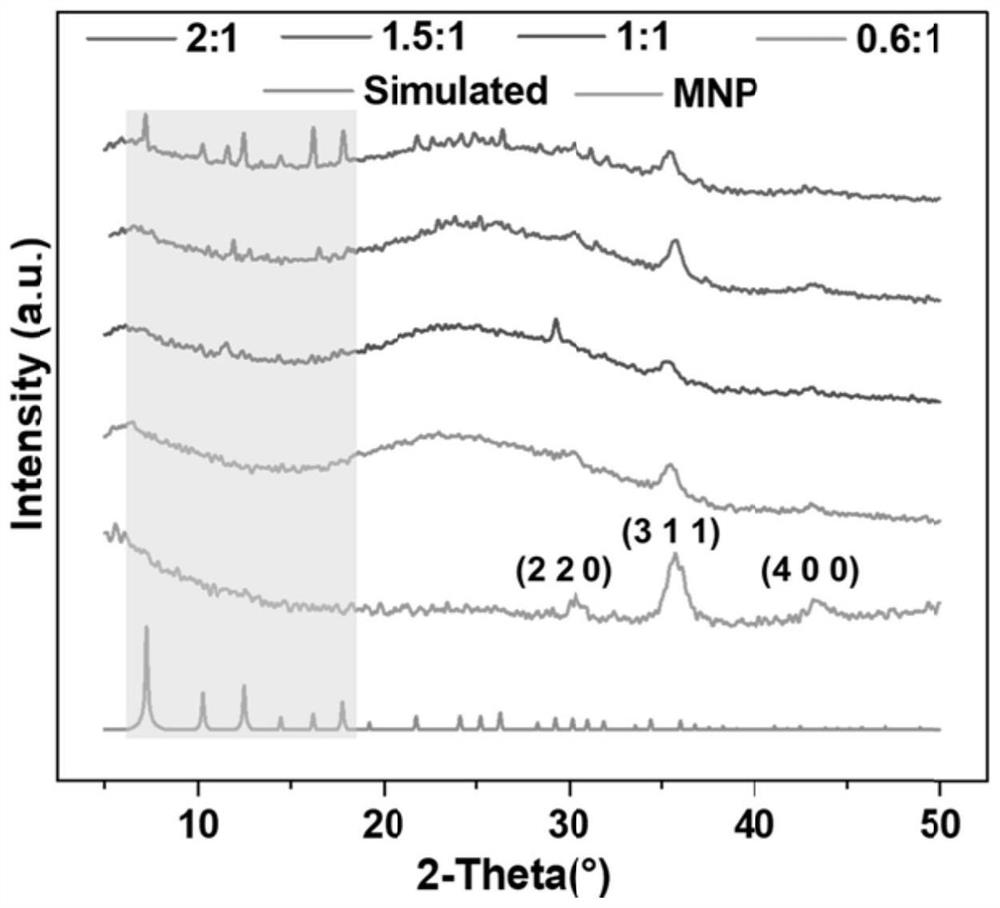
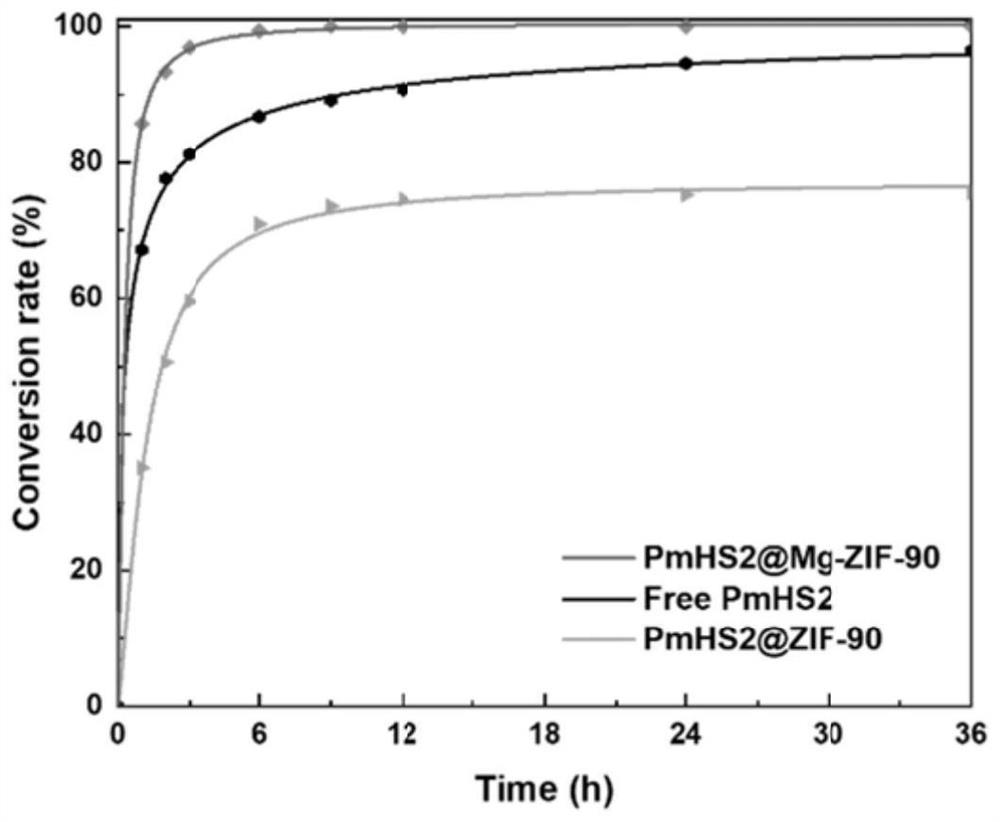
Magnetic nanoparticle-glycosyl transferase-amorphous metal organic framework composite catalytic material as well as preparation method and application thereof
PendingCN113980926ALarge apertureIncrease reaction rateTransferasesOn/in inorganic carrierMagnetite NanoparticlesMetal-organic framework
The invention discloses a magnetic nanoparticle-glycosyl transferase-amorphous metal organic framework composite catalytic material as well as a preparation method and application thereof. The composite catalytic material is formed by wrapping glycosyl transferase and magnetic nanoparticles in an amorphous metal organic framework as a novel immobilized enzyme material. After the composite catalytic material is prepared, the composite catalytic material is added into a mixed solution of a glycosyl donor and a glycosyl acceptor, and a heparin disaccharide product is obtained through a catalytic glycosylation reaction. The amorphous metal organic framework material has a larger aperture than a common metal organic framework, reduces mass transfer resistance, improves enzymatic efficiency, is hollow inside, and is more beneficial to maintenance of enzyme molecule activity. The composite catalytic material can be used for efficiently catalyzing the glycosylation reaction of monosaccharide, not only has good thermal stability and chemical stability, but also can improve the enzymatic reaction rate, can be recycled for multiple times, keeps higher catalytic activity and simplifies the separation step.
Owner:NANJING NORMAL UNIVERSITY
Synthetic method for 3,6-branched glucosepolyheptasaccharide
PendingCN108912239AActivate the immune system for self-defenseGood regulationNatural productAcid hydrolysis
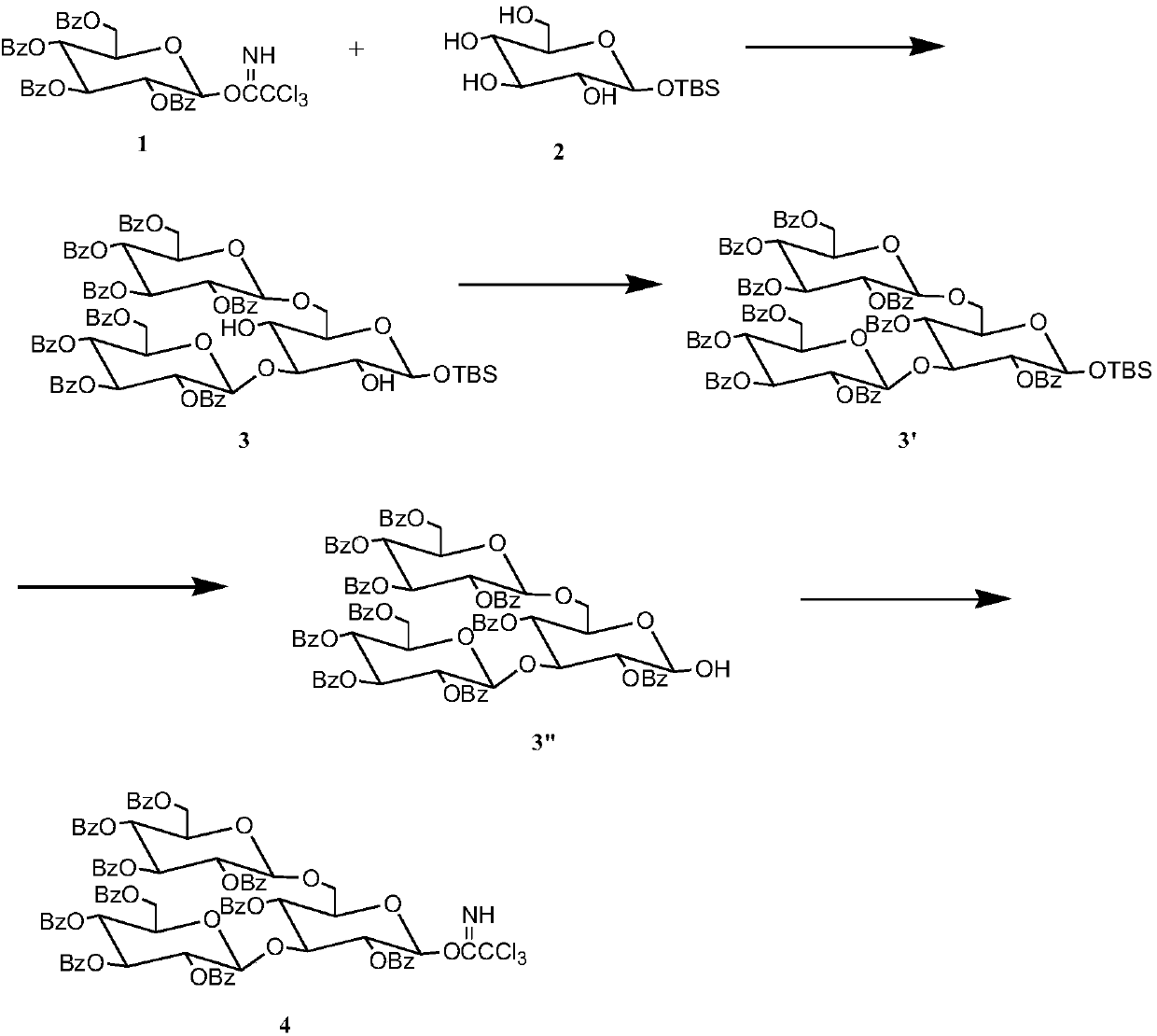
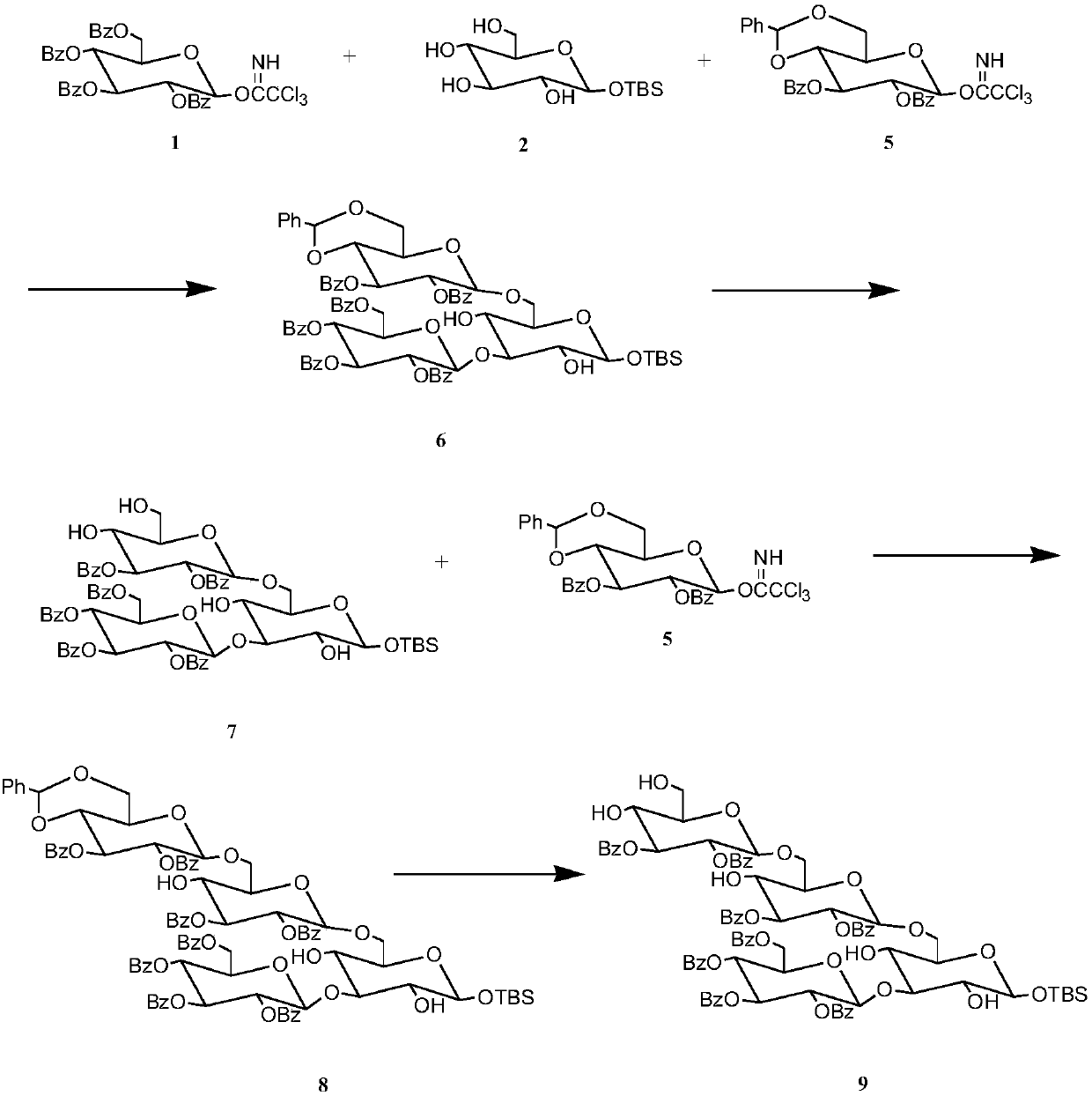
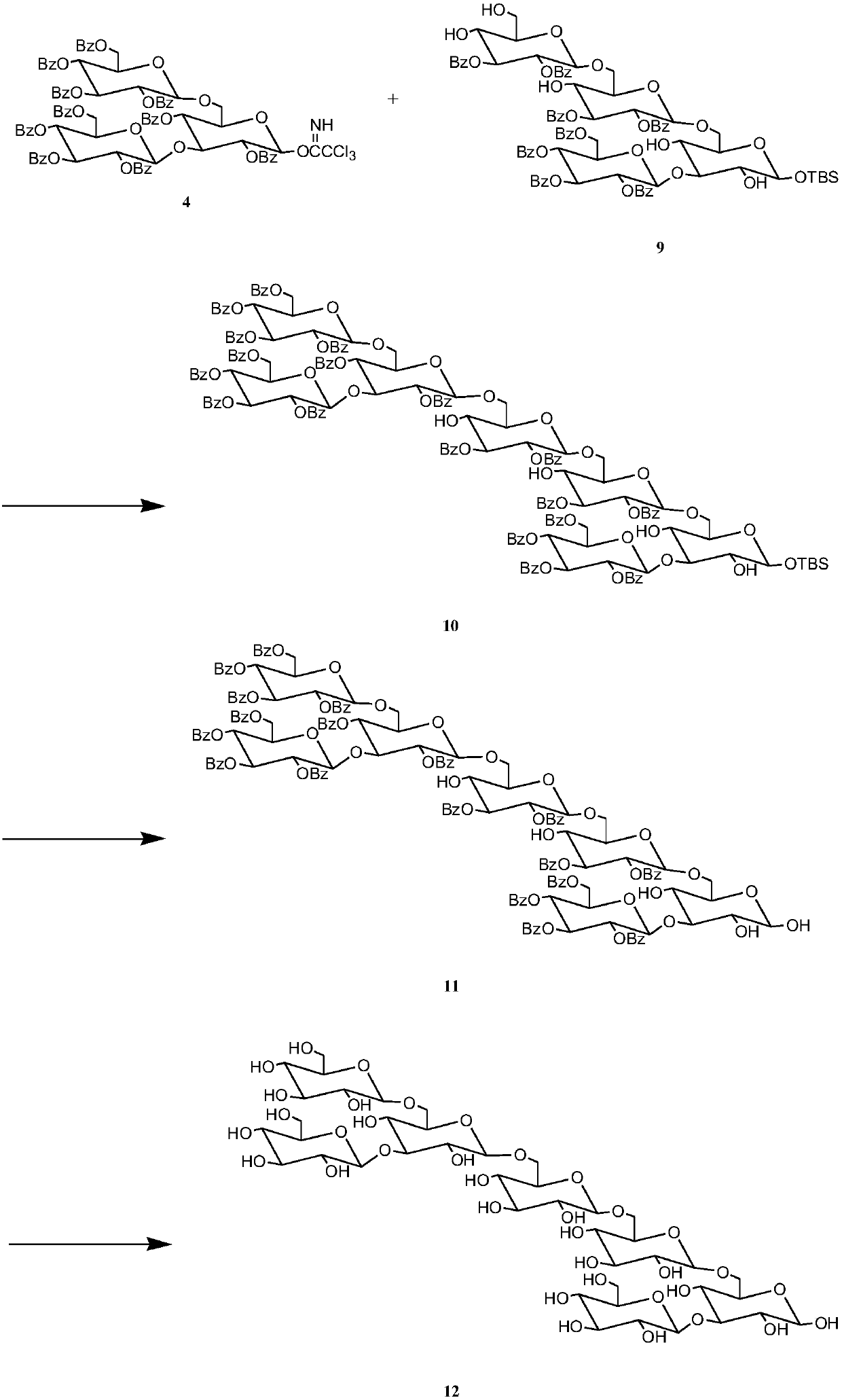
The invention discloses a synthetic method for 3,6-branched glucosepolyheptasaccharide, and belongs to the technical field of natural product synthesis. The method comprises the following steps: glucose trichloroacetimidate is used as a glycosyl donor, tert-butyldimethylsilyl-alpha-D-glucopyranose is used as a glycosyl acceptor, coupling is performed, acid hydrolysis is performed, activation is performed to obtain a glucose trisaccharide donor, glucose trichloroacetimidate and 4,6-phenylmethylene-2,3-di-O-benzoylglucose trichloroacetimidate are used as glycosyl donors, tert-butyldimethylsilyl-alpha-D-glucopyranose is used as a glycosyl acceptor, coupling is performed, hydrolysis is performed, the obtained material is used as a glycosyl acceptor, 4,6-phenylmethylene-2,3-di-O-benzoylglucosetrichloroacetimidate is used as a glycosyl donor, coupling is performed, hydrolysis is performed to obtain a glucose tetrasaccharide acceptor, the glucose trisaccharide donor is coupled with the glucose tetrasaccharide acceptor to obtain heptasaccharide, deprotection is performed, and therefore the target product 3,6-branched glucosepolyheptasaccharide is obtained. The synthesis disclosed by the invention is convenient to operate, has a high utilization rate of the raw materials, and is a high-efficiency synthetic method.
Owner:江西艾立斯特生物科技有限公司
Glycosylation reaction catalyst, glycosylation method and application
ActiveCN113926472AHigh yieldHigh stereoselectivityPhysical/chemical process catalystsSugar derivativesPtru catalystReceptor
The invention belongs to the technical field of sugar chemistry, and relates to a glycosylation reaction catalyst, a glycosylation method and application. The glycosylation method takes platinum chloride (IV) as a catalyst and is simple to operate, wide in substrate application range and high in reaction regioselectivity and stereoselectivity, and application of the method in glycosylation reaction is disclosed. According to the method, alpha-configuration O-glycosyl trichloroacetimidate is used as a donor, platinum (IV) chloride is used as a catalyst of an acid-base catalysis way, and a product mainly having a beta-configuration is obtained. In the method, a catalyst and a glycosyl acceptor are firstly combined to form a catalyst-acceptor adduct, so that proton acidity and oxygen affinity are increased, donor activation is enhanced, and acceptor transfer is carried out. According to the invention, the ligand binding capacity of platinum (IV) chloride and the characteristic that a diol receptor can be used as a bidentate ligand thereof are fully utilized, so that the method is not only suitable for construction of high-stereoselectivity glycosidic bonds of monohydroxyl receptors, but also can realize glycosidic bond construction with high regioselectivity and stereoselectivity of receptors of 1,3-diol, 1,2-cis-diol, 1,2-trans-diol, and 1,2,3-triol structures.
Owner:SHANDONG UNIV
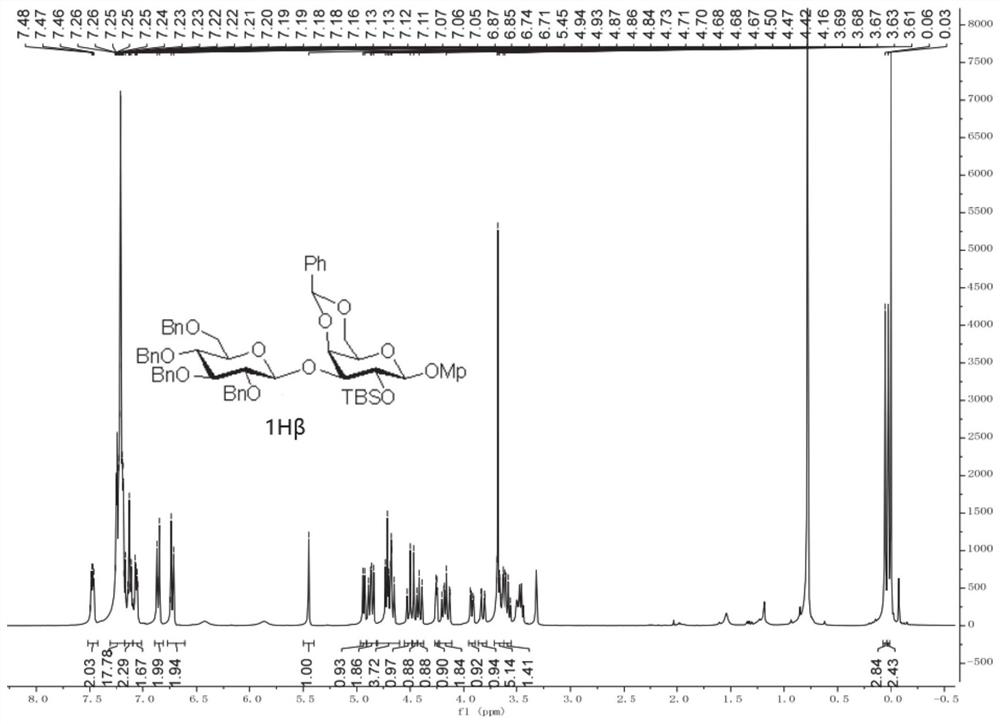
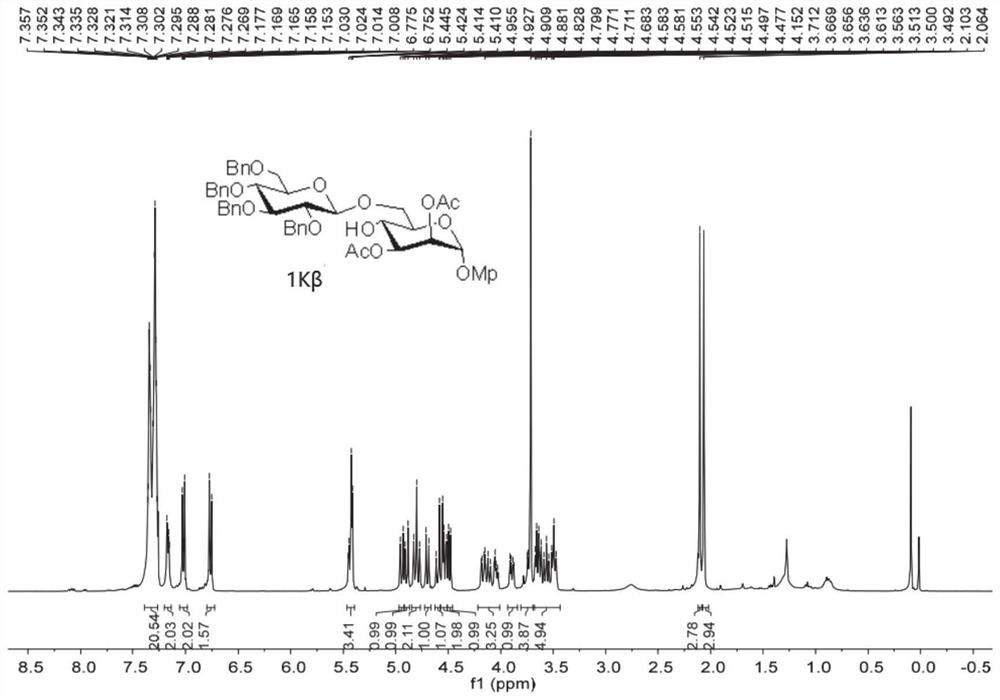
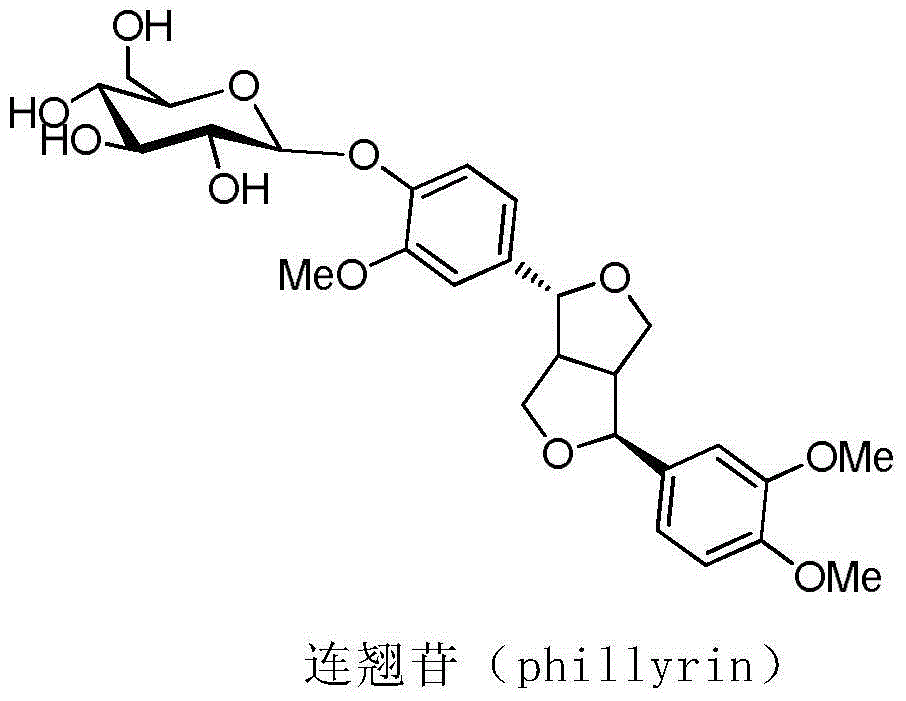
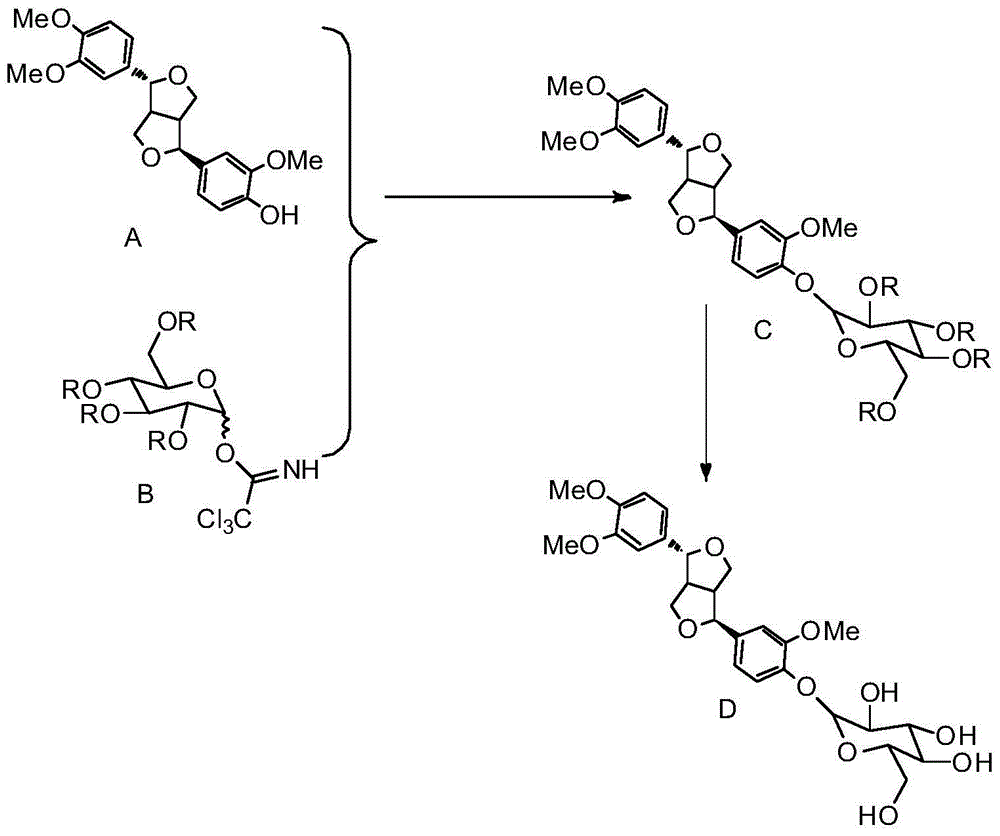
Chemical synthesis method of phillyrin
ActiveCN105461767AConvenient sourceReduce manufacturing costOrganic active ingredientsSugar derivativesChemical synthesisOrganic solvent
The present invention relates to a method for chemical synthesis of phillyrin. According to the method, a glycosyl acceptor phillygenin and a glycosyl donor are dissolved in an organic solvent, a glycosylation reaction is performed to prepare tetra acyl phillyrin, the tetra acyl phillyrin is dissolved in an organic solvent, sodium methylate is added, a deacylation reaction is performed, an acidic pH value adjusting agent is added to adjust the pH value of the reaction mixture to achieve a neutral state, and finally a purification treatment is performed to obtain the product. According to the present invention, the advanced and practical characteristics of the production method comprise convenient raw material source, cheap and easily-available catalyst for the glycosylation reaction, significantly-reduced preparation cost, and industrial production achieving.
Owner:富力
Stereoselective synthesis method of beta-2-deoxysugar, 2-deoxy-2-azide sugar and glucoside bond
PendingCN113527388AEasy to synthesizeEasy to installSugar derivativesOrganic chemistry methodsReceptorUronic acid
The invention discloses a stereoselective synthesis method of beta-2-deoxysugar, 2-deoxy-2-azide sugar or glucoside bond. According to the method, 2-diphenyl acetyl phosphino (DPPA) on a glycosyl donor is utilized, and a hydrogen-bond interaction is formed between phosphorus and oxygen and hydroxyl of a glycosyl receptor, so that a glycosidic bond with high surface selectivity is formed. According to the method, the stereoselectivity of glycosylation reaction can be efficiently controlled, and particularly, the method has huge advantages in synthesis of challenging beta-configuration 2-deoxysugar and 2-deoxy-2-azide glycoside. The method is wide in substrate application range, convenient to operate and suitable for synthesizing various saccharide molecules with biological activity. The DPPA group can be chemically and selectively removed under the mild catalytic action of Ni (OTf) 2 so that the possibility is provided for further synthesizing uronic acid or high-deoxy sugar.
Owner:CHINA PHARM UNIV
Synthetic method for 3,6-site branched glucohexaose
The invention discloses a synthetic method for 3,6-site branched glucohexaose and belongs to the technical field of natural product synthesis. The synthetic method comprises the following steps: coupling trichloroacetimidate of glucose as a glycosyl donor with tertiary butyl dimethyl silicon-alpha-D-glucopyranose as a glycosyl acceptor to generate trisaccharide; carrying out benzoyl protection andacid hydrolysis to remove end position protecting groups; then carrying out activation to obtain a glucotriose donor; coupling trichloroacetimidate of glucose and 4,6-benzylidenyl-2,3-di-O-benzoyl trichloroacetimidate of glucose as a glycosyl donor with tertiary butyl dimethyl silicon-alpha-D-glucopyranose as a glycosyl acceptor to synthesize trisaccharide; carrying out hydrolysis to remove 4,6-benzylidenyl to obtain the glucotriose acceptor; coupling the trisaccharide donor with the trisaccharide acceptor to obtain hexaose; and then carrying out de-protection to obtain the targeted 3,6-sitebranched glucohexaose. The synthetic method provided by the invention is concise in synthetic route and convenient to operate. In the whole process, the utilization ratio of raw materials is high, andthe method is an efficient synthetic method.
Owner:江西艾立斯特生物科技有限公司
Preparation method of carbohydrate derivative by utilization of glycosyl transferase
InactiveCN106755211AGood water solubilityImprove thermal stabilityFermentationSolubilityCarbohydrate derivative
The invention discloses a preparation method of a carbohydrate derivative by the utilization of glycosyl transferase. The preparation method comprises the following steps: adding glycosyl transferase into a solution with sugar concentration of 0.1-1 M to make the final activity concentration of a reaction vessel reach 0.1-3.0 U / mL, adding at least one glycosyl receptor selected from a group consisting of 2-amino acid, 3-amino acid, 4-amino acid, hydroquinone and arbutin into the reaction vessel of 15-50 DEG C to make the final activity concentration reach 0.01-3 wt% and carrying out a reaction at 28-37 DEG C for 1-18 h, letting glucose or fructosyl of sugar be transferred into the above receptor and finally acidifying with acetic acid so as to obtain the carbohydrate derivative. The invention discloses the derivative prepared by the above method. The derivative raises water-solubility, has efficacy of preventing decomposition or oxidation when contacting with light and heat, can be selectively transferred on glucose channels of cytomembrane of blood vessels and brain, and can be effectively used in relevant industries such as food, cosmetic, pharmaceuticals, etc.
Owner:BIOLAND BIOCHEM HAIMEN CO LTD
Thermostable beta-galactosidase and application thereof in synthesis of glycerol galactoside
PendingCN114774394AHigh yieldReduce consumption lossGenetic engineeringFermentationReceptorReaction temperature
The invention provides thermostable beta galactosidase and a related recombinant engineering strain thereof, and relates to the technical field of recombinase catalysis engineering. The beta galactosidase BtGal42 can be used for synthesizing glycerol galactoside by an enzymatic method at 50 DEG C by taking galactose as a glycosyl donor and taking glycerol as a glycosyl receptor, the enzyme stability is good at the temperature, and compared with the prior art, the use amount of the enzyme is obviously reduced, and the production cost is reduced. When the beta-galactosidase disclosed by the invention is used for a synthetic reaction, the bacterial contamination risk can be reduced and the reaction efficiency of a substrate can be improved by increasing the reaction temperature, so that the production efficiency of glyceryl galactoside is improved, and industrial application requirements can be completely met.
Owner:NANJING UNIV OF TECH +1
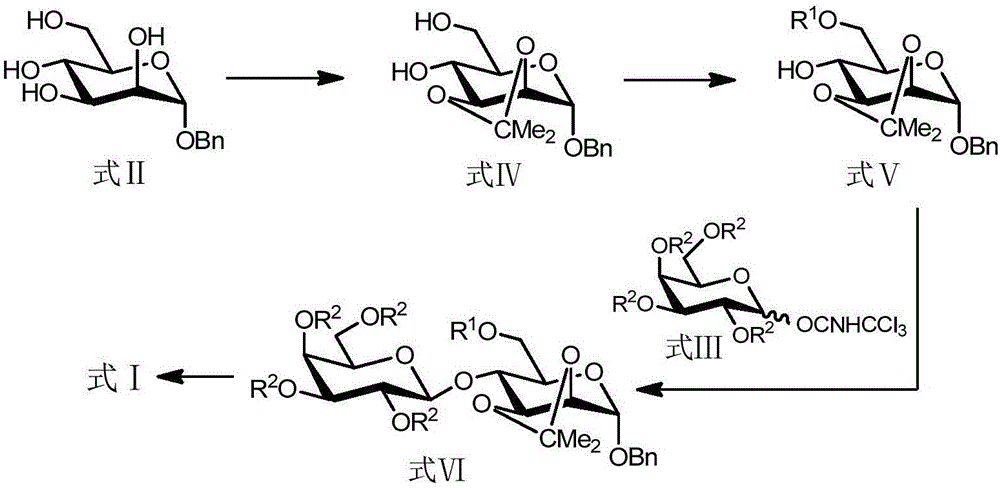
Batch synthesis method for 4-O-beta-Galactopyranosyl-D-mannopyranoside
The invention discloses a batch synthesis method for 4-O-beta-Galactopyranosyl-D-mannopyranoside. The batch synthesis method of 4-O-beta-Galactopyranosyl-D-mannopyranoside comprises the steps of 1) conducting the isopropylidenation of the compound shown in formula II with 2, 3-hydroxy to obtain the compound shown in formula IV; 2) protecting the 6-hydroxy of the compound shown in formula IV through an acylation reaction to obtain the compound shown in formula V; 3) carrying out a coupling reaction between the compound shown in formula V and the compound shown in formula III to obtain the compound shown in formula VI; 4) removing the isopropylidene protection, benzyl protection, R1 protection and R2 protection of the compound shown in formula VI to obtain the compound shown in formula I. The alpha-D-benzyl mannoside (formula II) serves as a starting material, glycosyl acceptor 2, 3-O-isopropylidene-6-O-benzoyl-alpha-D-pyran benzyl mannoside (formula V) intermediate is obtained through a two-steps reaction, the yield and purity in each step of the reaction are very high, accordingly, in a large-scale preparation, the intermediates IV and V do not require purification and can be directly used in the next step of the reaction.
Owner:CHINA AGRI UNIV
Glycosyl transferase-bimetal organic framework composite catalytic material, preparation method thereof and application of glycosyl transferase-bimetal organic framework composite catalytic material in synthesis of disaccharide and polysaccharide
PendingCN114426958AHigh catalytic activityImprove stabilityTransferasesFermentationMetal-organic frameworkPolysaccharide synthesis
The invention discloses a glycosyl transferase-bimetal organic framework composite catalytic material, a preparation method thereof and application of the glycosyl transferase-bimetal organic framework composite catalytic material in synthesis of disaccharide and polysaccharide, and the composite catalytic material is formed by taking a Zn-based MOFs material as a main body, doping non-Zn metal ions and wrapping glycosyl transferase in the Zn-based MOFs material. According to the composite catalytic material, a metal organic framework doped with bimetal ions serves as a novel immobilized enzyme material, glycosyl transferase is wrapped in the metal organic framework, and the glycosyl transferase-bimetal organic framework composite catalytic material is prepared and added into a glycosyl donor and a glycosyl acceptor, so that the glycosyl transferase-bimetal organic framework composite catalytic material is obtained. And catalyzing glycosylation reaction to obtain heparin disaccharide and polysaccharide. The composite catalytic material disclosed by the invention can efficiently catalyze the glycosylation reaction of monosaccharide, not only has good thermal stability and chemical stability, but also can improve the enzymatic reaction rate of immobilized enzyme compared with a common metal organic framework material, and can be recycled for multiple times while keeping higher catalytic activity.
Owner:NANJING NORMAL UNIVERSITY
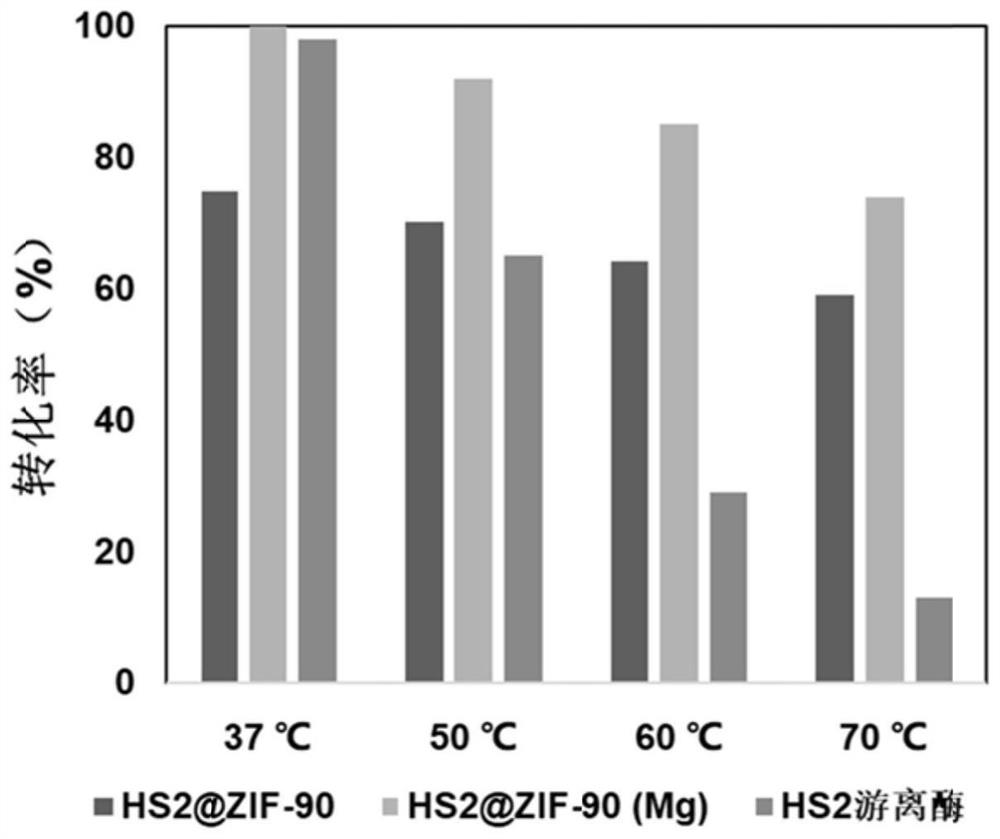
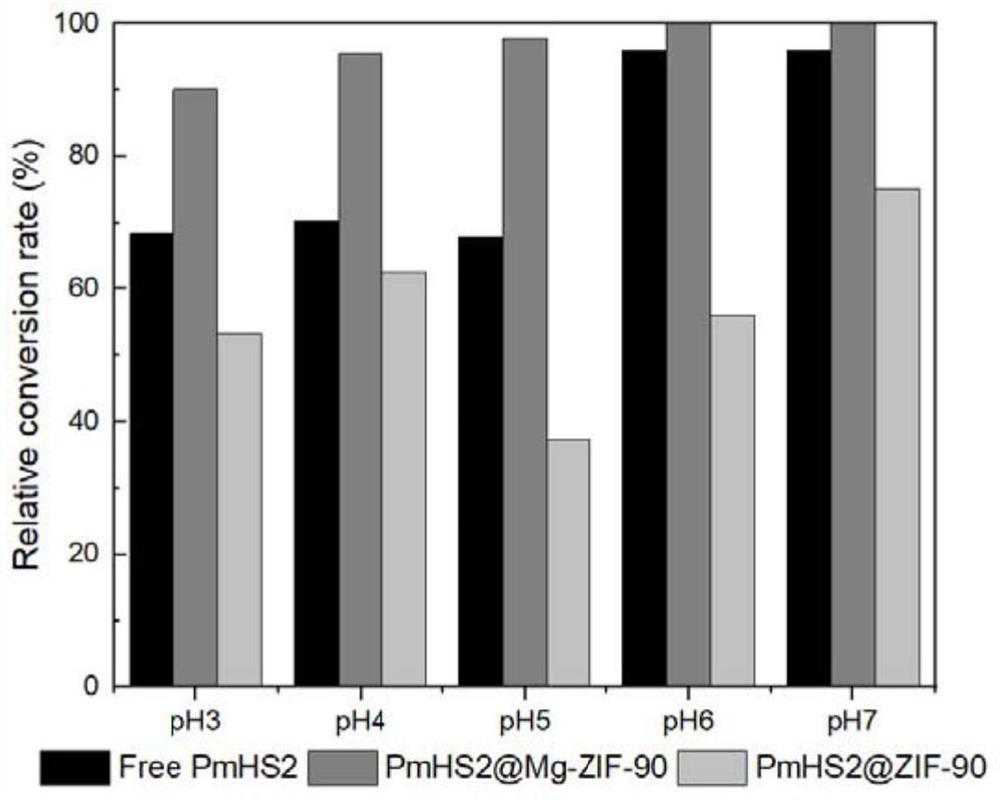
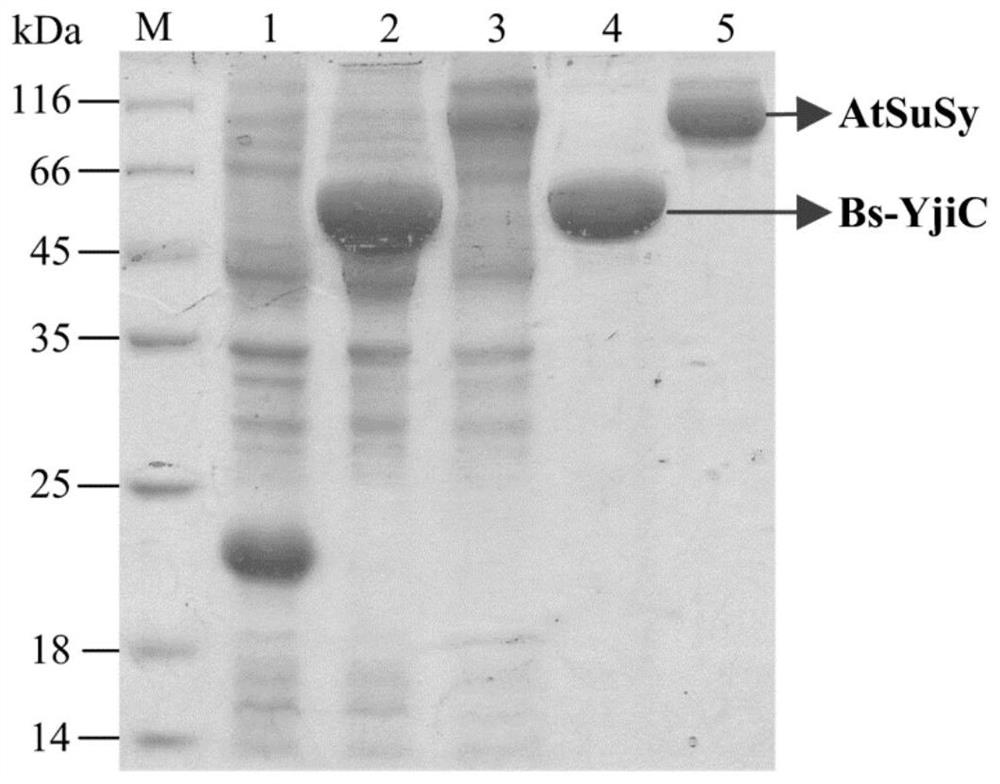
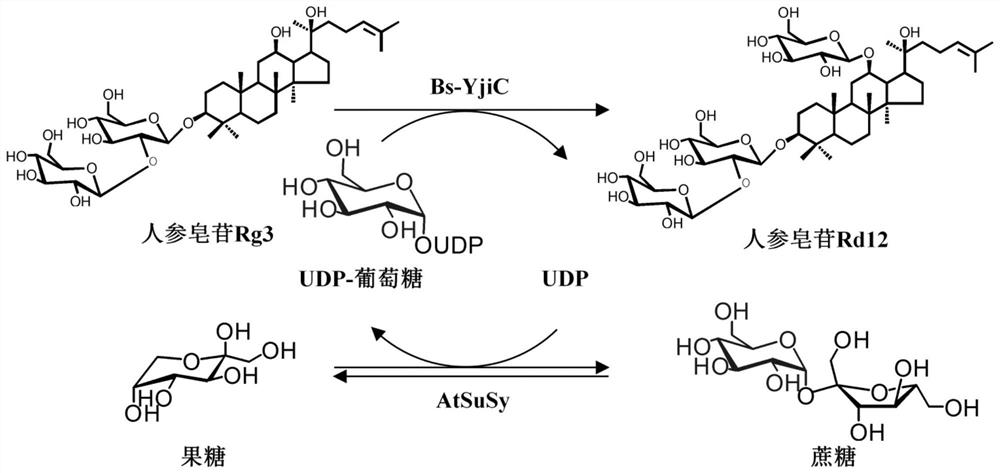
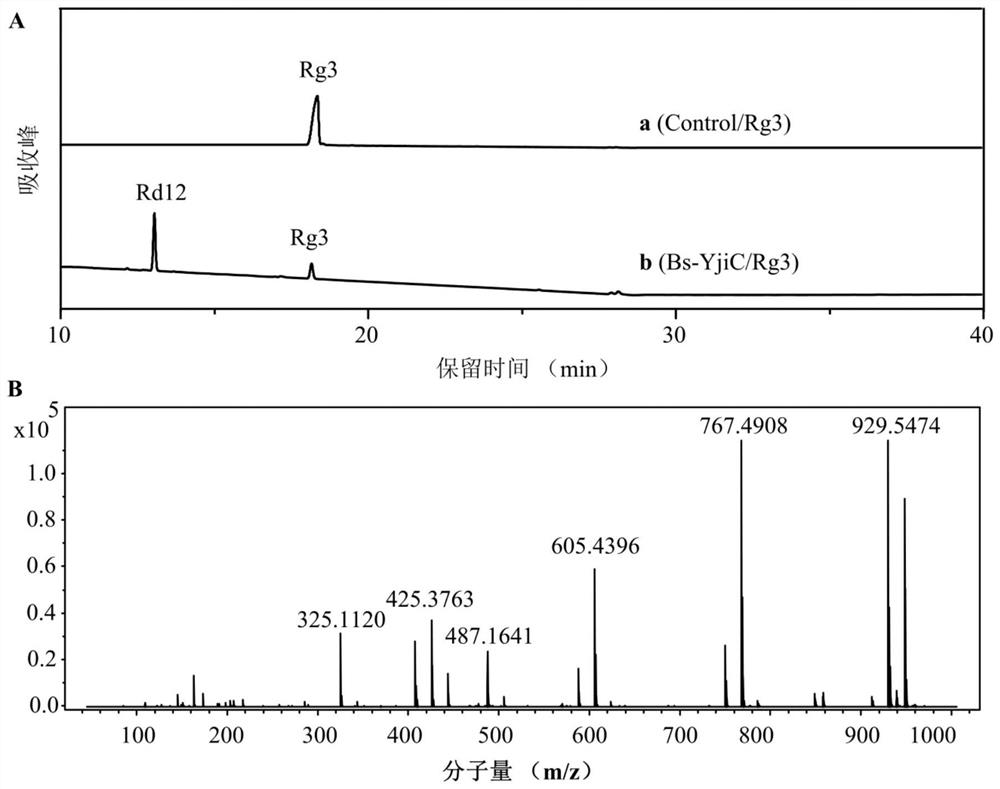
A kind of unnatural ginsenoside rd12 and its preparation method and application
ActiveCN107868115BEasy to purifyImprove conversion efficiencyOrganic active ingredientsSteroidsPtru catalystSucrose synthetase
The invention relates to a new unnatural ginsenoside Rd12 (3-O-β-D-glucopyranosyl (1-2)-β-D-glucopyranosyl-12-O-β-D-glucopyranosyl-protopanaxadiol) and a preparation method thereof and application, the present invention uses cheap sucrose as glucose glycosyl donor, utilizes glycosyltransferase and sucrose synthase as coupling catalyst, uses ginsenoside Rg3 as glycosyl acceptor, and uses cheap sucrose as glycosyl donor, Efficiently catalyze the C12 hydroxyl glycosylation of ginsenoside Rg3 to produce unnatural ginsenoside Rd12, which has the characteristics of good specificity, high catalytic efficiency, and simple product purification. In addition, the unnatural ginsenoside Rd12 prepared by the invention can be used as an antitumor drug, and the unnatural ginsenoside Rd12 has significant inhibitory effects on colon cancer cells, liver cancer cells, lung cancer cells and gastric cancer cells.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
A kind of synthetic method of the glycoside based on indoxyl derivative, 2-(benzothiazol-2'-yl)phenol derivative
ActiveCN106432369BEasy to operateHigh yieldSugar derivativesFluorescence/phosphorescenceThiazoleIndoxyl
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
A method for synthesizing various glycosides based on 4-methylumbelliferone
The invention discloses a method for synthesizing various glucosides on a basis of 4-methylumbelliferone. According to the invention, a glycosyl donor peracetyl saccharide and a glycosyl acceptor 4-methylumbelliferone are subjected to a glycosylation reaction under room temperature or under heating with dichloromethane or 1,2-dichloroethane as a solvent and with the combined effect of Lewis acid boron trifluoride ethyl ether and organic alkali triethylamine or pyridine; and protecting groups are removed, such that various glucosides based on 4-methylumbelliferone can be obtained. The glucosides include 4-methylumbelliferone-beta-D-glucopyranosiduronide, 4-methylumbelliferone-beta-D-glucopyranoside, 4-methylumbelliferone-beta-D-xylopyranoside, 4-methylumbelliferone-beta-D-ribofuranoside, 4-methylumbelliferone-alpha-D-galactopyranoside, and 4-methylumbelliferone-alpha-D-mannopyranoside. The method is simple, and can produce a beta or alpha single-configuration target. A glycosylation reaction yield can reach 17-93%.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
Beta-galactosidase capable of glycosylating resveratrol
ActiveCN103305491BBroaden the range of receptorsBacteriaMicroorganism based processesEnzyme proteinMolecular modification
Owner:SHANDONG UNIV
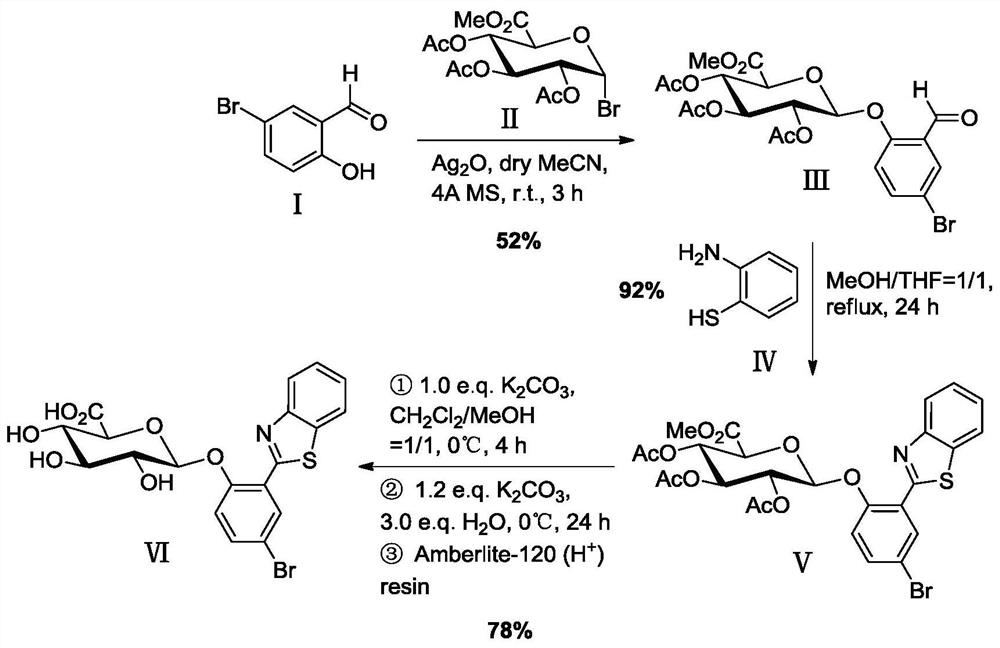
A kind of β-glucuronidase precipitation type fluorescent substrate synthesis method
ActiveCN109810157BHigh reaction conversion rateMild reaction conditionsSugar derivativesMicrobiological testing/measurementGlucuronidasePhenol
The invention discloses a method for synthesizing a β-glucuronidase precipitation-type fluorescent substrate based on 2-(benzothiazole-2'-yl)-4-bromophenol. The synthesis method comprises the following three-step reactions: 1) glycosylation reaction; 2) aromatic ring reaction; 3) deprotection group reaction. In the synthesis method, the reaction yield of each step can reach above the middle level, the highest is more than 90%, the reaction conversion rate of the material with relatively high price or preparation cost is relatively high, and the total yield of the three steps can reach 37%, and the reaction conditions are mild. Easy to implement. At the same time, the glycosylation reaction step and the deprotection group reaction step in the synthesis method can be applied to the synthesis of common fluorescent substrates 4-methylumbelliferyl-β-D with 4-methylumbelliferone as a glycosyl acceptor ‑Glucuronide, the two-step reaction yields can reach 51%, 89%, respectively, and the total yield can reach about 45%.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +2
Batch synthesis method of epilactose
The invention discloses a batch synthesis method for 4-O-beta-Galactopyranosyl-D-mannopyranoside. The batch synthesis method of 4-O-beta-Galactopyranosyl-D-mannopyranoside comprises the steps of 1) conducting the isopropylidenation of the compound shown in formula II with 2, 3-hydroxy to obtain the compound shown in formula IV; 2) protecting the 6-hydroxy of the compound shown in formula IV through an acylation reaction to obtain the compound shown in formula V; 3) carrying out a coupling reaction between the compound shown in formula V and the compound shown in formula III to obtain the compound shown in formula VI; 4) removing the isopropylidene protection, benzyl protection, R1 protection and R2 protection of the compound shown in formula VI to obtain the compound shown in formula I. The alpha-D-benzyl mannoside (formula II) serves as a starting material, glycosyl acceptor 2, 3-O-isopropylidene-6-O-benzoyl-alpha-D-pyran benzyl mannoside (formula V) intermediate is obtained through a two-steps reaction, the yield and purity in each step of the reaction are very high, accordingly, in a large-scale preparation, the intermediates IV and V do not require purification and can be directly used in the next step of the reaction.
Owner:CHINA AGRI UNIV
Oligosaccharide synthesizing method
ActiveCN106674302ASimple structureEasy to storeSugar derivativesSugar derivatives preparationState of artOrganic solvent
The invention discloses an oligosaccharide synthesizing method which is characterized in that full-benzyl protection propargyl alcohol glycoside is utilized as a glycosyl donor, 6-site glycosyl intermediate or glycosyl with a -Tr protecting group is utilized as a glycosyl acceptor, and the glycosyl donor and the glycosyl acceptor are dissolved in an organic solvent according to a mole ratio of (1 to 6mmol) to 1mmol to (0.1 to 1mmol) to perform glycosylation reaction under the catalysis of ferric salt. Compared with the prior art, the oligosaccharide synthesizing method achieves the purpose that the glycosyl donor directly perform glycosylation reaction with the 6-site glycosyl intermediate with the -Tr protecting group, oligosaccharide is synthesized through a one-pot method in high yield, a reaction route is shortened, operation steps are simplified, production cost is reduced, and heavy metal ion pollution is effectively avoided. In addition, the oligosaccharide synthesizing method has the advantages of being green, environment-friendly and suitable for industrial production and having larger technological innovation and good application prospect.
Owner:EAST CHINA NORMAL UNIV
Iterative oligosaccharide synthesis
ActiveCN100432085CEliminate complicated separation processThe synthesis steps are simpleSugar derivativesOligosaccharidesOligosaccharide synthesisGlycosyl acceptor
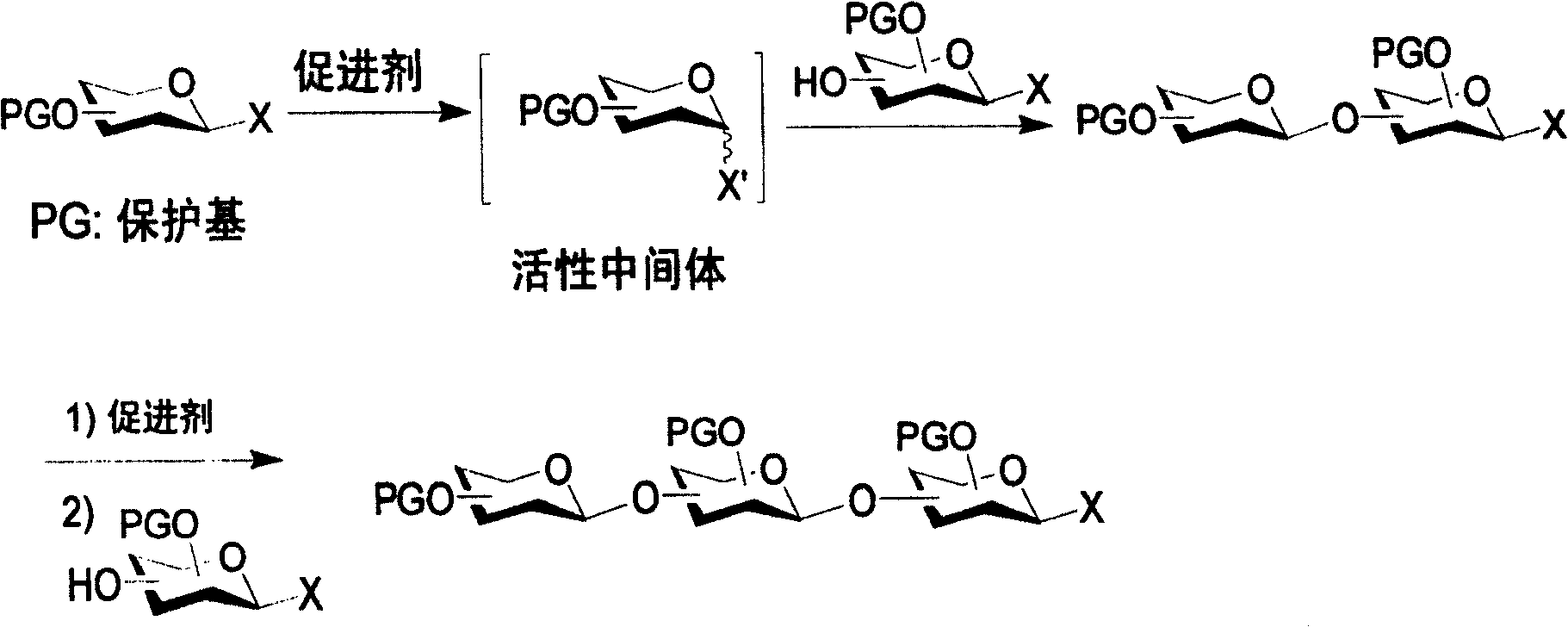
A process for synthesizing an oligosaccharide includes (a) activating a protected glycosyl donor with a promoter in the absence of a glycosyl acceptor to produce a reactive intermediate, the glycosyl donor having an activatable aglycon at the anomeric carbon; (b) adding a protected glycosyl donor / acceptor to the reactive intermediate to produce a new glycosyl donor, the glycosyl donor / acceptor having both an activatable aglycon at the anomeric carbon and a free hydroxyl group; (c) repeating steps (a) and (b) to add any additional protected glycosyl donor / acceptors; and (d) adding a protected glycosyl acceptor to produce the oligosaccharide, the glycosyl acceptor having a free hydroxyl group and a non-activatable aglycon at the anomeric carbon.
Owner:PEKING UNIV
A kind of cyclodextrin glucosyltransferase mutant and its application
ActiveCN110804597BIncrease productionStrong specificityBacteriaFermentationCyclodextrinGlycosyl acceptor
The invention discloses a cyclodextrin glucosyltransferase mutant and application thereof, belonging to the technical fields of enzyme engineering and microorganism engineering. The CGTase mutant of the present invention has high product specificity for long-chain glycosylated genistein; use the CGTase mutants A156V / L174P, A156V / L174P / A166Y, A156V / L174P / A166V, A156V / L174P / A166G and A156V of the present invention / L174P / A166K using maltodextrin as a glycosyl donor and genistein as a glycosyl acceptor to produce long-chain glycosylated genistein has a higher yield than wild-type cyclodextrin glucosyltransferase As a glycosyl donor, the production of long-chain glycosylated genistein with genistein as a glycosyl acceptor increased by 62.5%, 165%, 112.5%, 112.5% and 59.4%, respectively.
Owner:JIANGNAN UNIV
Fructose glycosylated curcumin as well as preparation method and application thereof
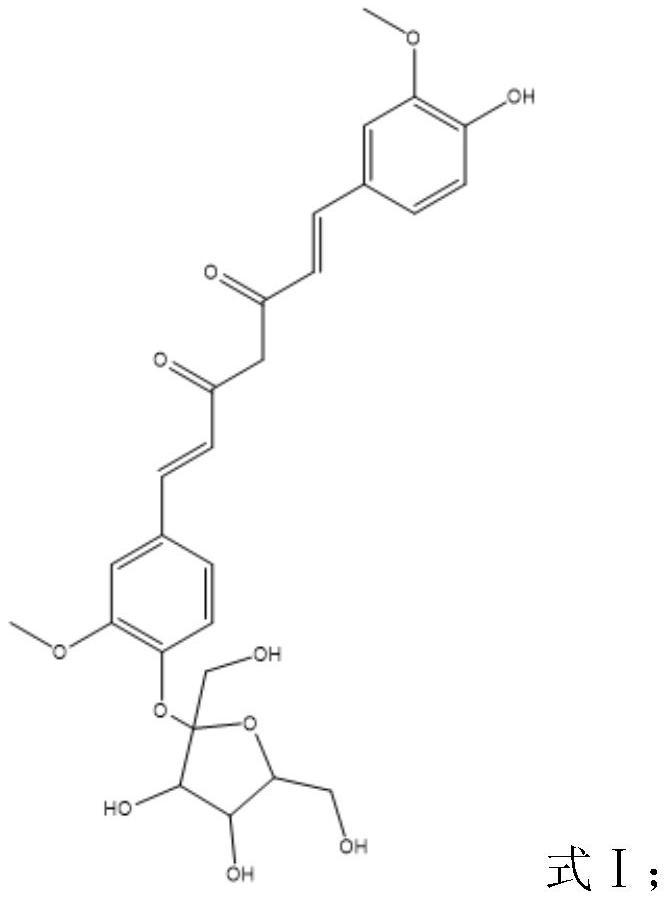
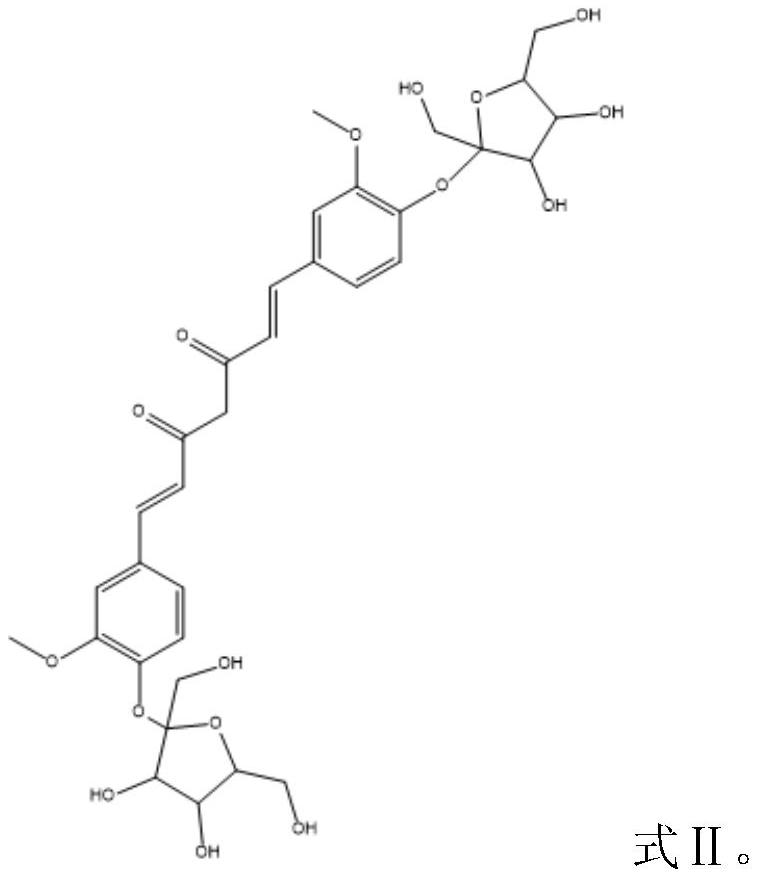
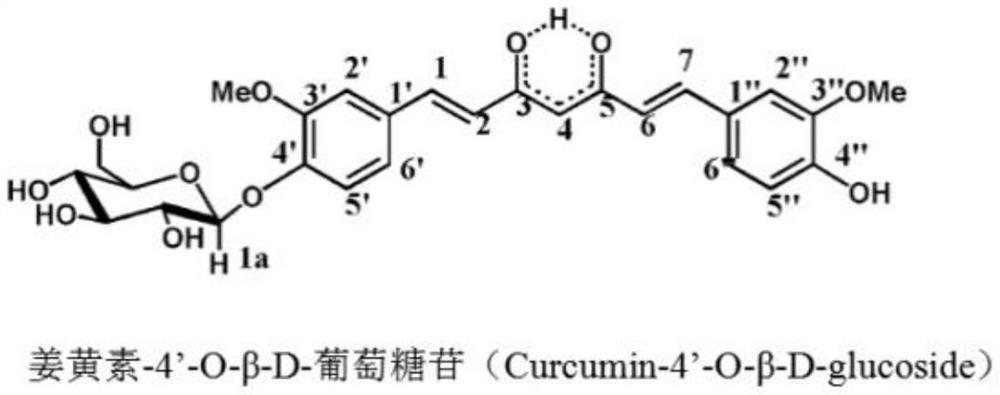
PendingCN114790472AHas the advantage of industrializationReduce manufacturing costOrganic active ingredientsNervous disorderFructanGlycosyl acceptor
The invention discloses curcumin subjected to fructose glycosylation, a preparation method and application, levan sucrase is used as a catalyst, curcumin is used as a glycosylation acceptor, cane sugar is used as a glycosylation donor to carry out enzymatic glycosylation reaction, the curcumin subjected to fructose glycosylation is obtained, the solubility of the curcumin subjected to fructose glycosylation is far higher than that of the curcumin subjected to glucose glycosylation, and the curcumin subjected to fructose glycosylation can be used for preparing the curcumin subjected to fructose glycosylation. Therefore, the better bioavailability is achieved.
Owner:上海龙殷生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com