Method for preparing allolactose
A technology of glucofuranose and acetyl, which is applied in the field of allolactose preparation, can solve the problems of poor reproducibility, impossibility of purification, and low yield, and achieve the effects of good stereoselectivity, easy purification, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
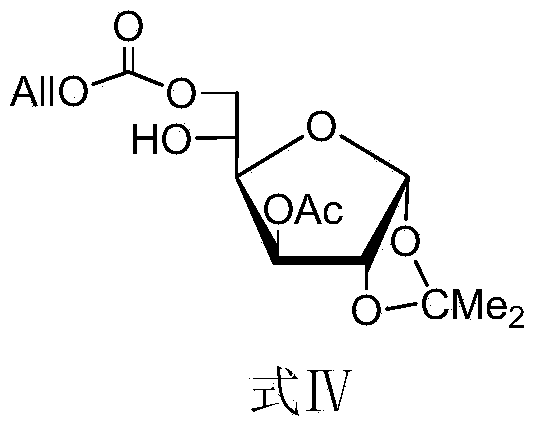
[0067] Synthesis of compound shown in embodiment 1, formula IV
[0068]
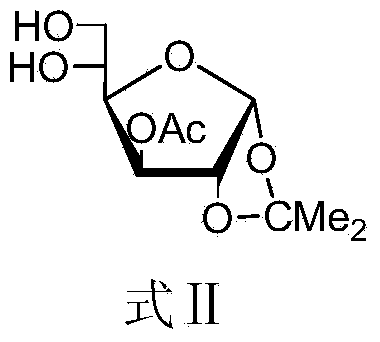
[0069] Dissolve the compound (7.86 g, 30.0 mmol) shown in Formula II in 100 mL of dry CH 2 Cl 2 Add Py (12mL, 150.0mmol), cool in an ice bath to -10°C, add AllocCl (3.55mL, 33mmol) in 40mL of CH 2 Cl 2 Solution. Remove the ice bath after the dropwise addition, and stir at room temperature for 2 hours. TLC (3:1, petroleum ether-ethyl acetate) detected that the reaction was complete. The reaction system was sequentially washed with 1M hydrochloric acid and saturated NaHCO 3 solution, washed with saturated brine, CH 2 Cl 2 Extraction, anhydrous Na 2 SO 4 Drying, concentration, and column chromatography (4:1, petroleum ether-ethyl acetate) separated the compound represented by formula IV (10 g, yield 97%).
[0070] Structural Confirmation Data:
[0071] [α] D 25 =+108°(1g / 100mL, CHCl 3 ), 1 H NMR (CDCl 3 ,300MHz)δ:1.31,1.51(2s,6H,CMe 2 ),2.13(s,3H,COCH 3 ),2.94(d,J=4.5Hz,1H,OH),3.90(m,1H,H...
Embodiment 2
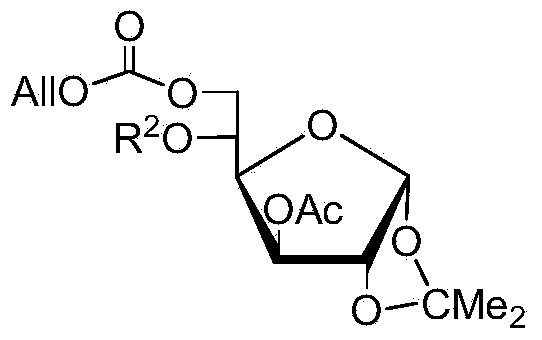
[0073] Embodiment 2, the synthesis of compound 2 (belonging to a kind of general formula compound V)
[0074]
[0075] Dissolve the compound shown in Formula IV (10.4g, 30mmol) in 60mL of dry pyridine, add BzCl (4.2mL, 36mmol) dropwise under ice bath, stir for 30 minutes under ice bath after the addition, and then place the system at room temperature After reacting for 5 hours, TLC (3:1, petroleum ether-ethyl acetate) detected that the reaction was complete. The reaction system with CH 2 Cl 2 Dilute with 1M hydrochloric acid, saturated NaHCO 3 solution, washed with saturated brine, CH 2 Cl 2 Extraction, anhydrous Na 2 SO 4 Drying and concentration column chromatography (4:1, petroleum ether-ethyl acetate) separated to give light yellow viscous liquid 2 (13.5g, yield 100%).
[0076] Structural Confirmation Data:
[0077] [α] D 25 =+120°(1g / 100mL, CHCl 3 ), 1 H NMR (CDCl 3 ,300MHz)δ:1.31,1.52(2s,6H,CMe 2 ),2.01(s,3H,COCH 3 ),4.40(dd,J=5.0Hz,12.2Hz,1H,H-4),4.51(...
Embodiment 3
[0079] Embodiment 3, the synthesis of compound 3 (belonging to a kind of general formula compound VI)
[0080]
[0081] In a 250mL round bottom flask, dissolve compound 2 (9g, 20mmol) in 200mL methanol-ethyl acetate (volume ratio, 1 / 2) solution, add CH to the system 3 COONH 4 (30.8g, 400mmol), cooled in an ice bath to –10°C, and quickly added PdCl to the system 2 (35mg, 0.2mmol), NaBH 4 (1.52g, 40mmol). TLC (2:1, petroleum ether-ethyl acetate) detected that the reaction was complete, and it took 1 hour in total. The reaction solution was concentrated under reduced pressure to remove methanol and tetrahydrofuran. The reaction system with CH 2 Cl 2 Diluted, extracted with saturated saline, anhydrous Na 2 SO 4 Drying and concentration column chromatography (4:1, petroleum ether-ethyl acetate) separated to give light yellow viscous liquid 3 (6.9 g, yield 95%).
[0082] Structural Confirmation Data:
[0083] [α] D 25 =+88°(1g / 100mL, CHCl 3 ), 1 H NMR (CDCl 3 ,300M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com