A kind of unnatural ginsenoside rd12 and its preparation method and application
A ginsenoside, non-natural technology, applied in the fields of biotechnology and botany, can solve problems such as high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
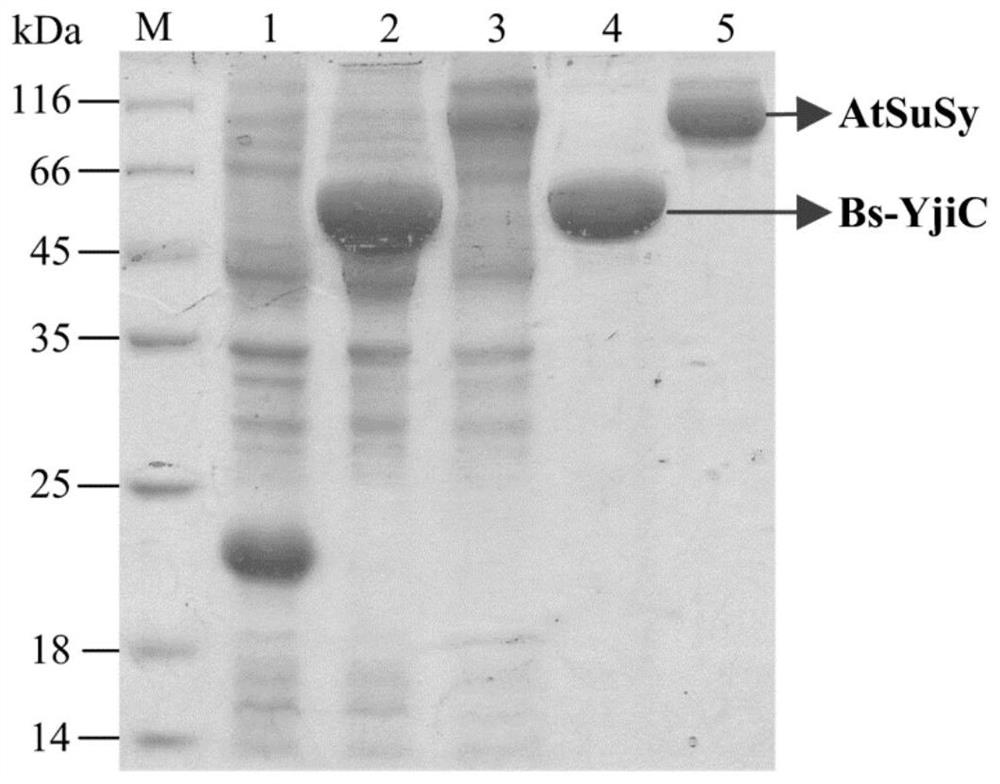
[0060] Cloning, expression and purification of embodiment 1 glycosyltransferase and sucrose synthase
[0061] 本发明专利中的糖基转移酶Bs-YjiC筛选自枯草芽孢杆菌(Bacillus subtilis168),其氨基酸序列如SEQ ID NO.1所示,其核酸序列(SEQ ID NO.7)如下:ATGAAAAAGTACCATATTTCGATGATCAATATCCCGGCGTACGGACATGTCAATCCTACGCTTGCTTTAGTAGAGAAGCTTTGTGAGAAAGGGCACCGTGTCACGTACGCGACGACTGAGGAGTTTGCGCCCGCTGTTCAGCAAGCCGGTGGAGAAGCATTGATCTATCATACATCCTTGAATATTGATCCTAAGCAAATCAGGGAGATGATGGAAAAGAATGACGCGCCCCTCAGCCTTTTGAAAGAATCACTCAGCATTCTGCCGCAGCTTGAGGAGTTATATAAGGATGATCAGCCTGATCTGATCATCTATGACTTTGTTGCGCTGGCTGGTAAATTGTTTGCTGAAAAGCTTAATGTTCCGGTCATTAAGCTCTGTTCGTCATATGCCCAAAATGAATCCTTTCAGTTAGGAAATGAAGACATGCTGAAAAAAATAAGAGAAGCAGAGGCTGAATTTAAAGCCTACTTGGAGCAAGAGAAGTTGCCGGCTGTTTCATTTGAACAGTTAGCTGTGCCGGAAGCATTAAATATTGTCTTTATGCCGAAGTCTTTTCAGATTCAGCATGAGACGTTCGATGACCGTTTCTGTTTTGTCGGCCCCTCTCTCGGAGAACGGAAGGAAAAAGAAAGCCTGTTGATTGACAAGGATGATCGCCCGCTTATGCTGATTTCTTTGGGTACGGCGTTTAACGCATGGCCGGAATTTTACAAGATGTGCATCAAGGCATTTCGGGATTCTTCATGGCAAGTGATCATGTCGGTTGGGAAAACGATTGATCCAGAAAG...
Embodiment 2
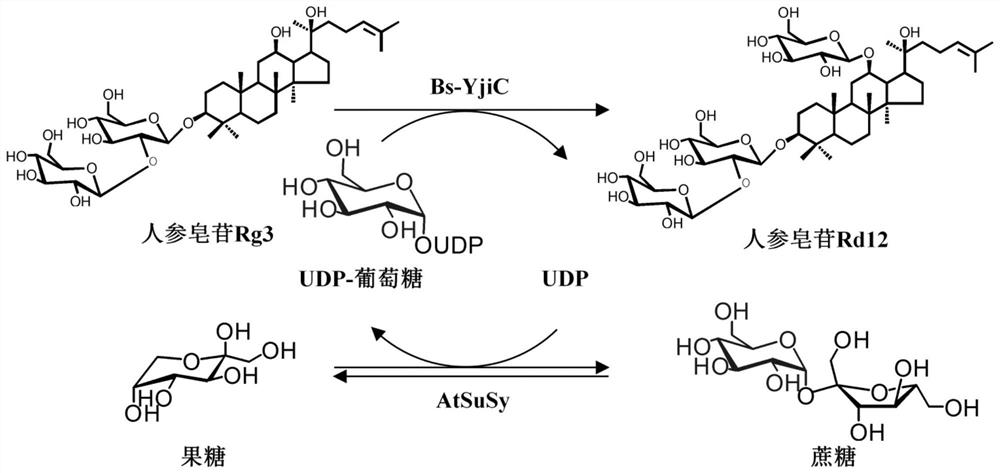
[0073] Example 2 Glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy are coupled to catalyze the synthesis of unnatural ginsenoside Rd12 from ginsenoside Rg3
[0074] Using ginsenoside Rg3 (3-O-[β-D-glucopyranosyl(1-2)-β-D-glucopyranosyl]-20(S)-protopanaxadiol) as substrate and cheap sucrose as glycosyl donor, by Glycosyltransferase-sucrose synthase double-enzyme coupling reaction can catalyze the C12-OH glycosylation of ginsenoside Rg3 to generate unnatural ginsenoside Rd12 (3-O-[β-D-glucopyranosyl(1-2)-β -D-glucopyranosyl]-12-β-D-glucopyranosyl-protopanaxadiol), the specific reaction is as figure 2 As shown, the details are as follows:
[0075]
[0076] The enzyme reaction system includes: 2mM Rg3, 0.5mM UDP, 300mM sucrose, 160U / mL glycosyltransferase Bs-YjiC and 200U / mL sucrose synthase AtSuSy, react at 35°C for 0.5h.
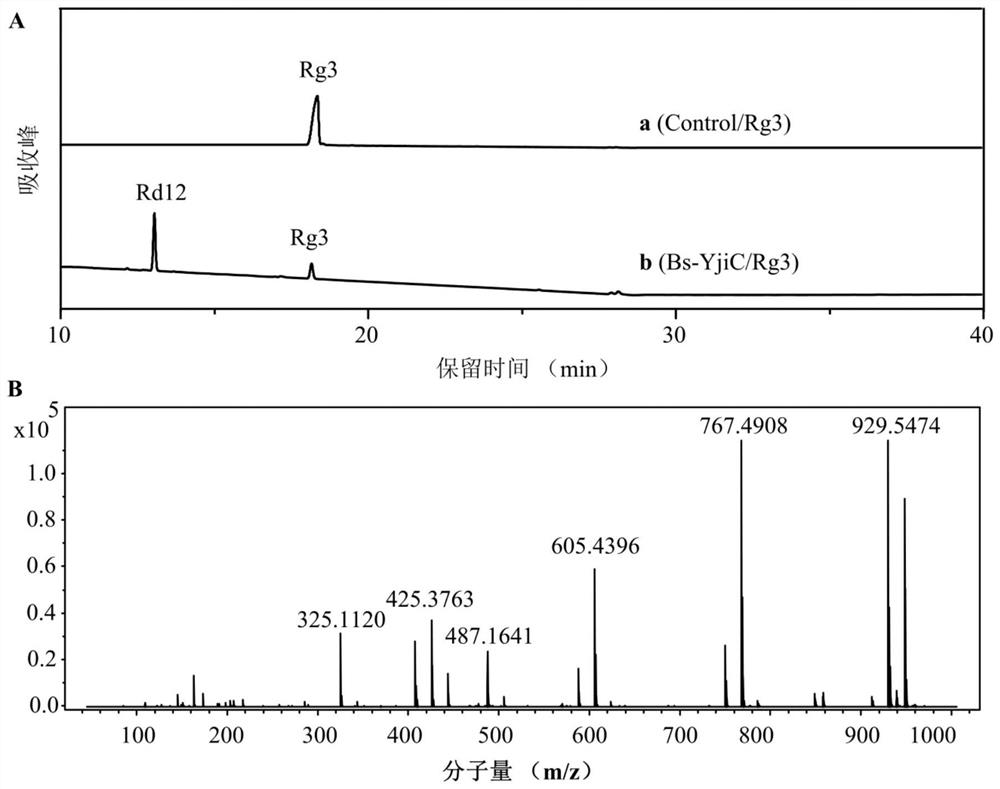
[0077] The enzyme reaction product was identified by high performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS...
Embodiment 3
[0079] Example 3 Fed-batch Synthesis of Unnatural Ginsenoside Rd12
[0080] In order to avoid the inhibition of glycosyltransferase activity by adding too much ginsenoside Rg3 at one time, and in view of the coupling reaction of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy within 0.5h, 2mM ginsenoside Rg3 sugar Kylation produces unnatural ginsenoside Rd12. Therefore, by feeding batches, 2mM ginsenoside Rg3 was supplemented at time points 1h, 2h, 4h and 8h to synthesize higher concentrations of non-natural ginsenoside Rd12.
[0081] From Figure 4 It can be seen that after 4 batches of supplementing ginsenoside Rg3 substrates and reacting for 18 hours, 9.8mM ginsenoside Rd12 (9.3g / L) can be finally obtained, the conversion rate of ginsenoside Rg3 reaches 98%, and the synthesis of ginsenoside Rd12 The rate reaches 0.5g / L / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com