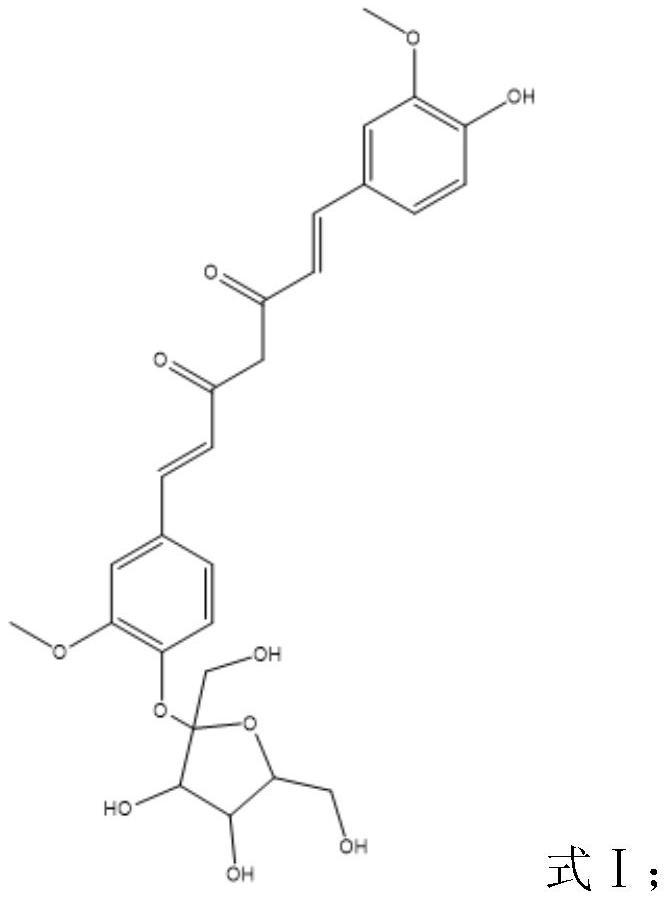
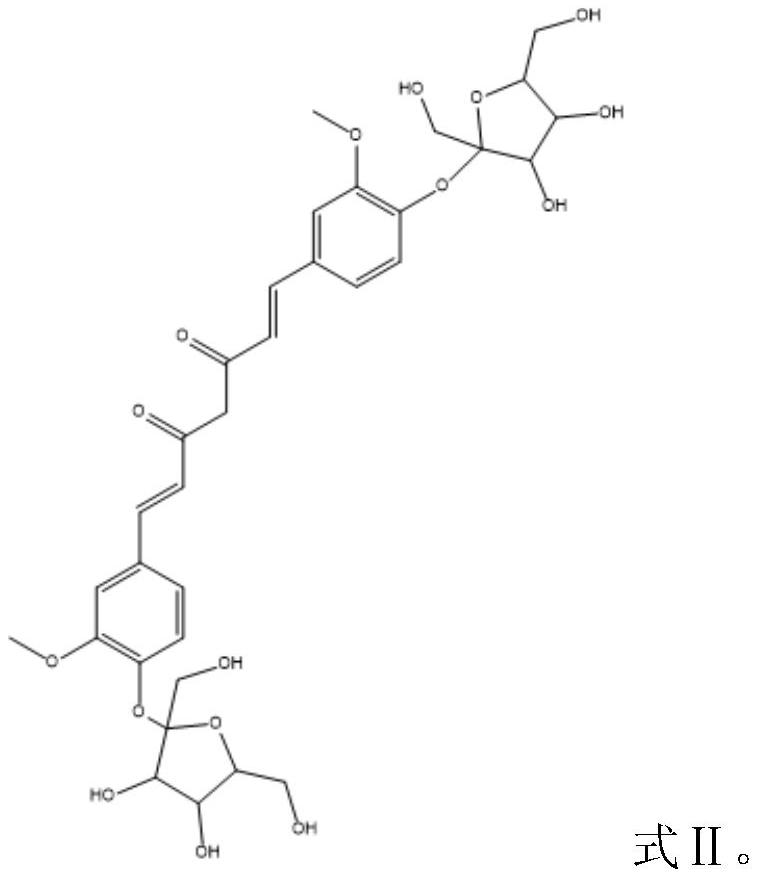
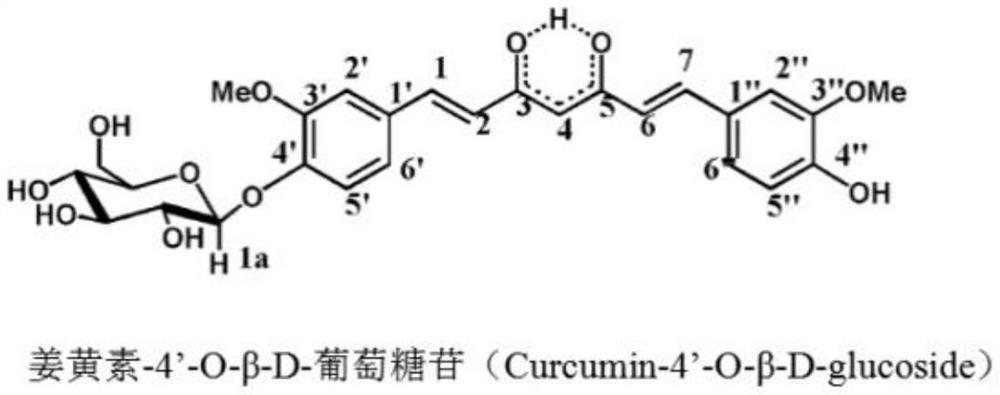
Fructose glycosylated curcumin as well as preparation method and application thereof
A technology of curcumin and glycosylation, which is applied in the direction of medical preparations containing active ingredients, organic chemistry, sugar derivatives, etc., to achieve the effect of industrialization advantages, low production costs, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Heterologous expression and purification of fructansucrase.
[0032] The fructansucrase LS used in this example is from Clavibactermichiganensis, and the nucleic acid and protein sequences of the enzyme are from the public NCBI gene database (https: / / www.ncbi.nlm.nih .gov / ), the nucleic acid and protein sequences of the enzyme can be obtained from the NCBI database with reference number (NCBI Reference Sequence: WP_011931834.1). According to the codon preference of Escherichia coli, it was codon-optimized, and a 6X His tag sequence was added to the end of its sequence, and the final sequence was sequence 1. The codon-optimized fructansucrase LS gene sequence was synthesized and subcloned into the E. coli expression vector pET30a(+) (Suzhou Jinweizhi Company, pET30a(+) is a published commercial E. coli expression vector). The recombinant plasmid pET30a-LS carrying the fructansucrase gene was transformed into E. coli BL21(DE3) host and screened overnight on LB-...
Embodiment 2
[0036] Example 2 Enzymatic fructoglycosylation of curcumin.
[0037] Taking the fructansucrase prepared in Example 1 as a catalyst, using curcumin (CAS: 458-37-7, purity 98%, Dingrui Chemical (Shanghai) Co., Ltd.) as a glycosyl acceptor, and using sucrose as a sugar The alkylation donor undergoes an enzymatic glycosylation reaction. In addition, several
[0038] The enzyme-catalyzed reaction conditions are as follows: 0.2M citrate sodium acetate buffer (pH 4.5-5.5) or 0.2M PBS (pH6.5-8.5) is used as the catalytic solvent system. The reaction was added with 0.5g curcumin (1.36mmol) as a glycosyl acceptor, 10g sucrose as a glycosyl donor, 1g fructansucrase, and 1g sophorolipid as a solubilizer to dissolve and disperse in a 100mL catalytic dissolving system, and The reaction was carried out in a constant temperature shaking water bath at 35°C for 6h.
[0039] The content of glycosylated curcumin in the catalytic solution after the reaction was analyzed by HPLC-MS / MS. The chro...
Embodiment 3
[0047] Example 3 Fructose glycosylated curcumin solubility
[0048] The reaction solution obtained by the reaction after proportional amplification of the fructose glycosylated curcumin sample preparation conditions described in Example 2 was used to prepare fructose glycosylated curcumin. Fructose glycosylated curcumin was separated and purified by Isolera rapid preparative chromatography, and the separation was carried out using SNAP KP-Sil (340 g) column. The separation process is to evenly add a sufficient amount of reaction solution to the sample cup, then place it in a vacuum drying oven at 60°C to evaporate the solvent, and then directly install the sample cup instead of the flow guide on the SNAP KP-Sil chromatographic column. Then proceed according to the chromatographic elution conditions: the elution flow rate is 8mL / min, the elution mobile phase is a binary mixture of methanol-0.1% acetic acid aqueous solution, and the elution gradient is 0-30min: methanol 15%→30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com