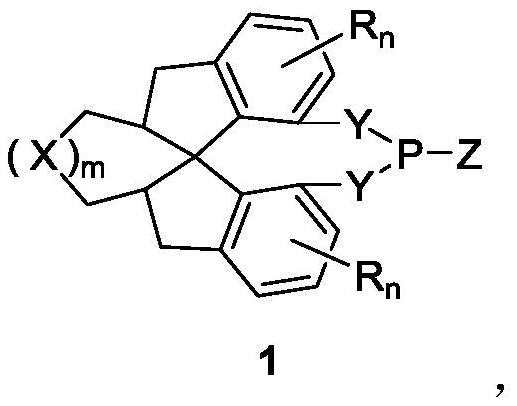
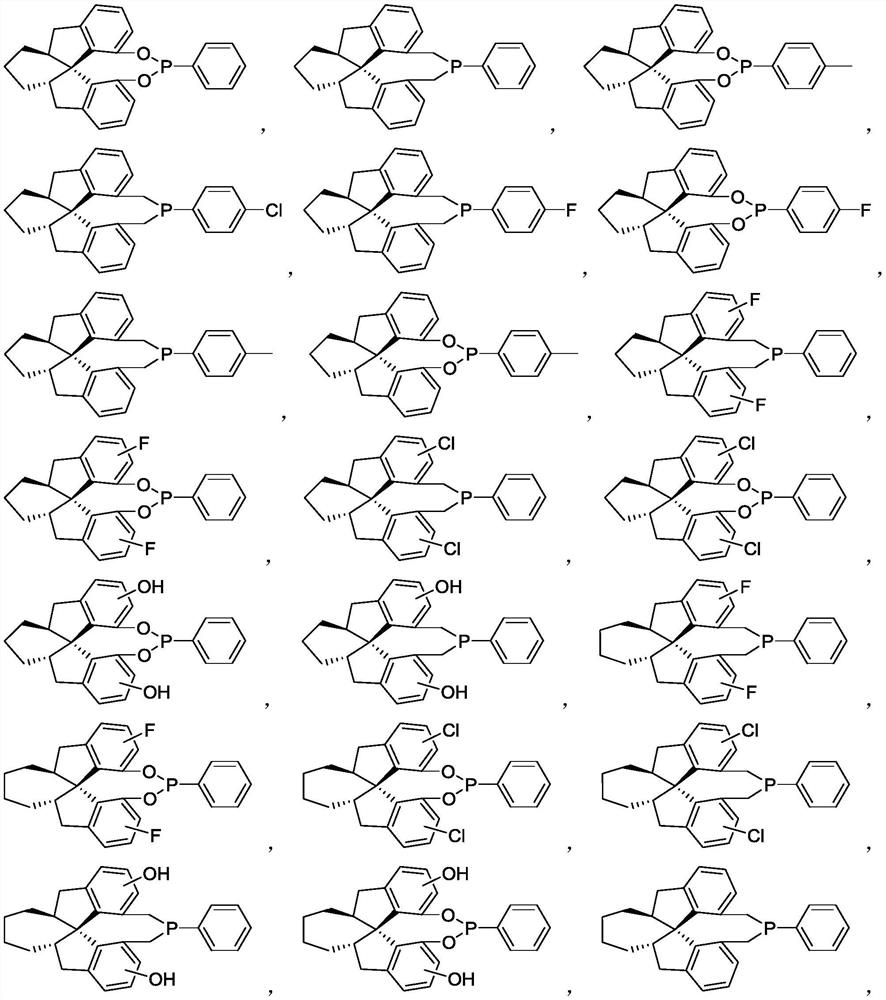
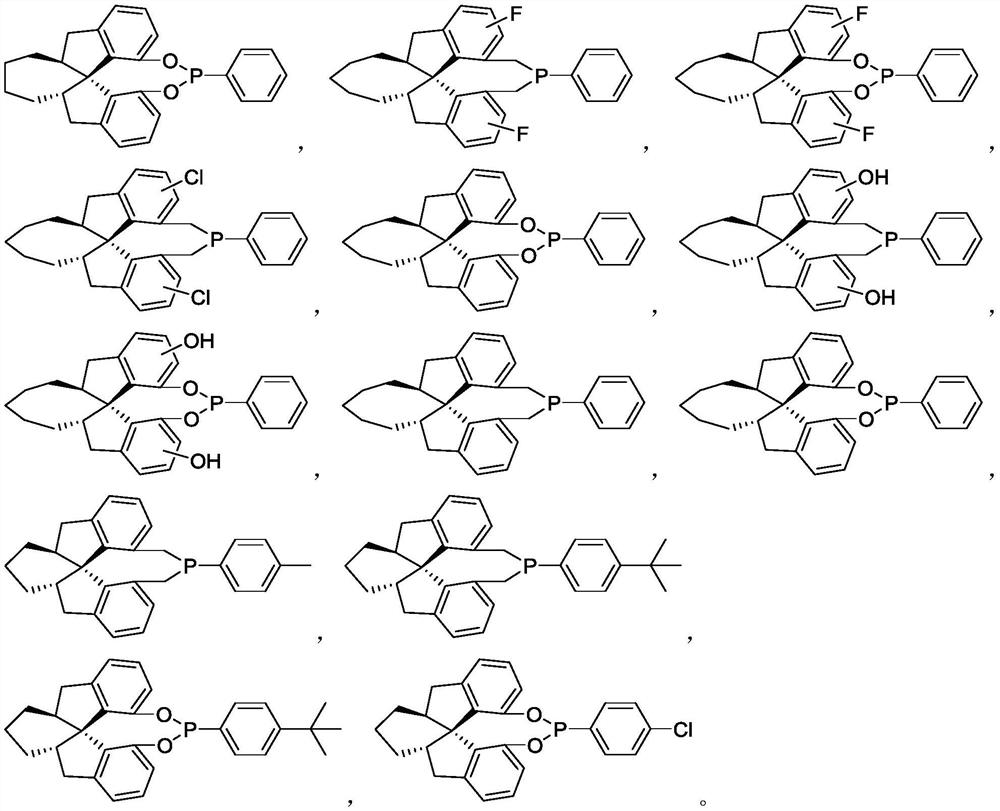
Chiral spiro monophosphine ligand and preparation method thereof
A spiro ring, monophosphine technology, applied in the field of organic synthesis, can solve problems such as less catalyst system, achieve the effects of high yield, optical purity, and significant technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Embodiment 1: the preparation of compound 3'-5
[0161]
[0162] Add compound 3'-6 (20.0g, 60mmol), pyridine (14.1mL, 175.0mmol) and 200mL fresh distilled CH in the 500mL reaction flask 2 Cl 2 , Add trifluoromethanesulfonic anhydride (25.5 mL, 150 mmol) dropwise at 0°C, after the addition is complete, stir at room temperature overnight. Quenched with water, CH 2 Cl 2 After extraction and drying over anhydrous magnesium sulfate, the reaction solution was concentrated and filtered through a silica gel column to obtain 30 g of yellow solid compound 3'-5 (eluent: n-hexane / ethyl acetate=10 / 1), yield: 90% .
[0163] 1 H NMR (400MHz, CDCl 3 )δ1.54-1.61(m,4H),1.67-1.72(m,2H),2.85-2.92(m,2H),2.93-2.99(m,2H),3.08-3.14(m,2H),7.10- 7.12 (m, 2H), 7.26-7.30 (m, 4H) ppm.
Embodiment 2
[0164] Embodiment 2: the preparation of compound 3'-4
[0165]
[0166] Add compound 3'-5 (21.4g, 38.4mmol), Pd(PPh 3 ) 4 (4.4g, 3.8mmol), Zn(CN) 2 (10.5g, 89.7mmol) and 100mL of anhydrous DMF, the reaction system was replaced with nitrogen, the oil bath was heated to 45°C, and the reaction was completed for 16 hours. TLC showed that the reaction was complete. Carefully added sodium hypochlorite solution to the reaction solution until the starch potassium iodide test paper was purple. Add 200 mL of ethyl acetate for extraction, wash with saturated sodium chloride, and dry over anhydrous sodium sulfate. After the reaction solution is concentrated, it is filtered through a silica gel column to obtain 11.5 g of off-white solid compound 3'-4 (eluent: n-hexane / ethyl acetate = 5 / 1), yield: 96%.
[0167] 1 H NMR (400MHz, CDCl 3 )δ1.55-1.69(m,6H),2.90-3.05(m,4H),3.15-3.20(m,2H),7.26-7.35(m,2H),7.45-7.47(d,J=7.6Hz, 2H),7.52-7.54(m,2H)ppm. 13 C NMR (100MHz, CDCl 3 ) δ16.6, 24...
Embodiment 3
[0168] Embodiment 3: the preparation of compound 3'-3
[0169]
[0170] In the 1000mL reaction flask, add compound 3'-4 (14.5g, 46.7mmol), 12M H 2 SO 4 (174mL), 6MHOAc (87mL), water (260mL), the oil bath was heated to reflux, and the reaction was monitored by TLC until the reaction of the raw materials was complete. Added 300mL of water, extracted with ethyl acetate (300mL×3), combined the organic phases, and washed with sodium hydroxide The aqueous solution was back-extracted, and the final concentrated hydrochloric acid was adjusted to a pH of about 3, extracted with ethyl acetate, dried, concentrated, and slurried with n-hexane to obtain a light brown solid. Further purification by column chromatography gave 10.5 g of white solid compound 3'-3, yield: 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com