Synthetic method for 3,6-site branched glucohexaose
A synthesis method and glucose technology, applied in the field of synthesis of 3,6-position branched hexaglucan, can solve the problems of unfavorable industrial production, complex synthesis method, low utilization rate of raw materials, etc., and achieve convenient operation, simple synthesis route, The effect of high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
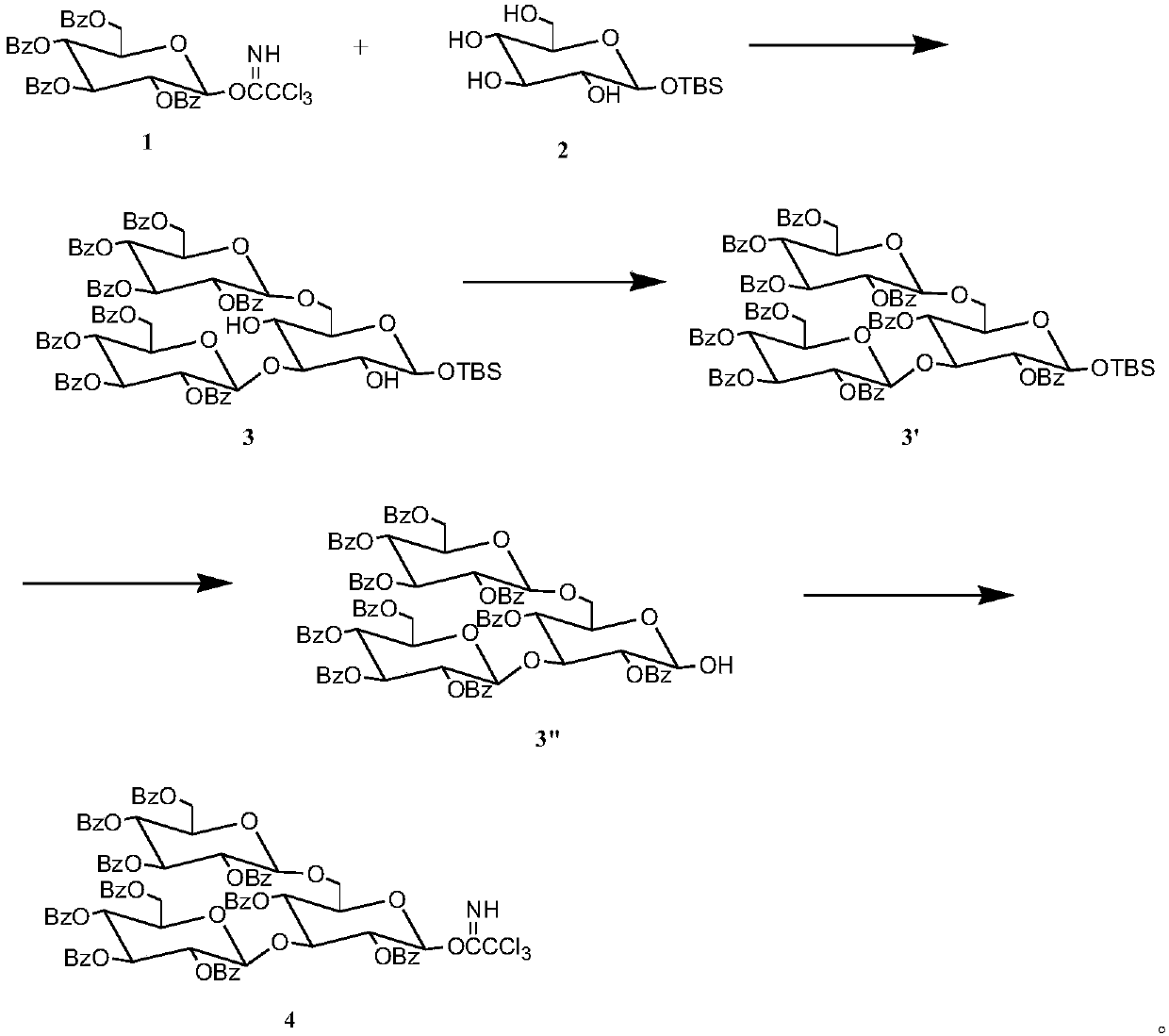
[0020] Preparation of glucotriose donor:
[0021] 1) Dissolve 3.705g, 5mmol of benzoylglucose trichloroacetimidate in 30mL of dichloromethane to obtain solution A, and dissolve 0.735g, 2.5mmol of tert-butyldimethylsilyl-α-D-glucopyranose Dissolve in 15mL of dichloromethane to obtain solution B, mix B and A to obtain solution C, add 230μL, 2mmol of TMSOTf catalyst to C, and then add Molecular sieves, after reacting at 25°C for 4 hours, thin-layer chromatography analysis showed that the reaction was complete, filtered with suction, evaporated the solvent under reduced pressure, separated by column chromatography, and used ethyl acetate / cyclohexane (1 / 3) as eluent Rinse and collect the corresponding components to obtain pure trisaccharides with a yield of 91.3%;
[0022] 2) Dissolve 7.250g, 5mmol trisaccharide in 30mL DMF, heat to 70°C to completely dissolve the glucose, add 2.9mL, 3.5mmol pyridine, take out the flask and cool it to 25°C, place the flask in an ice bath, add dro...
Embodiment 2
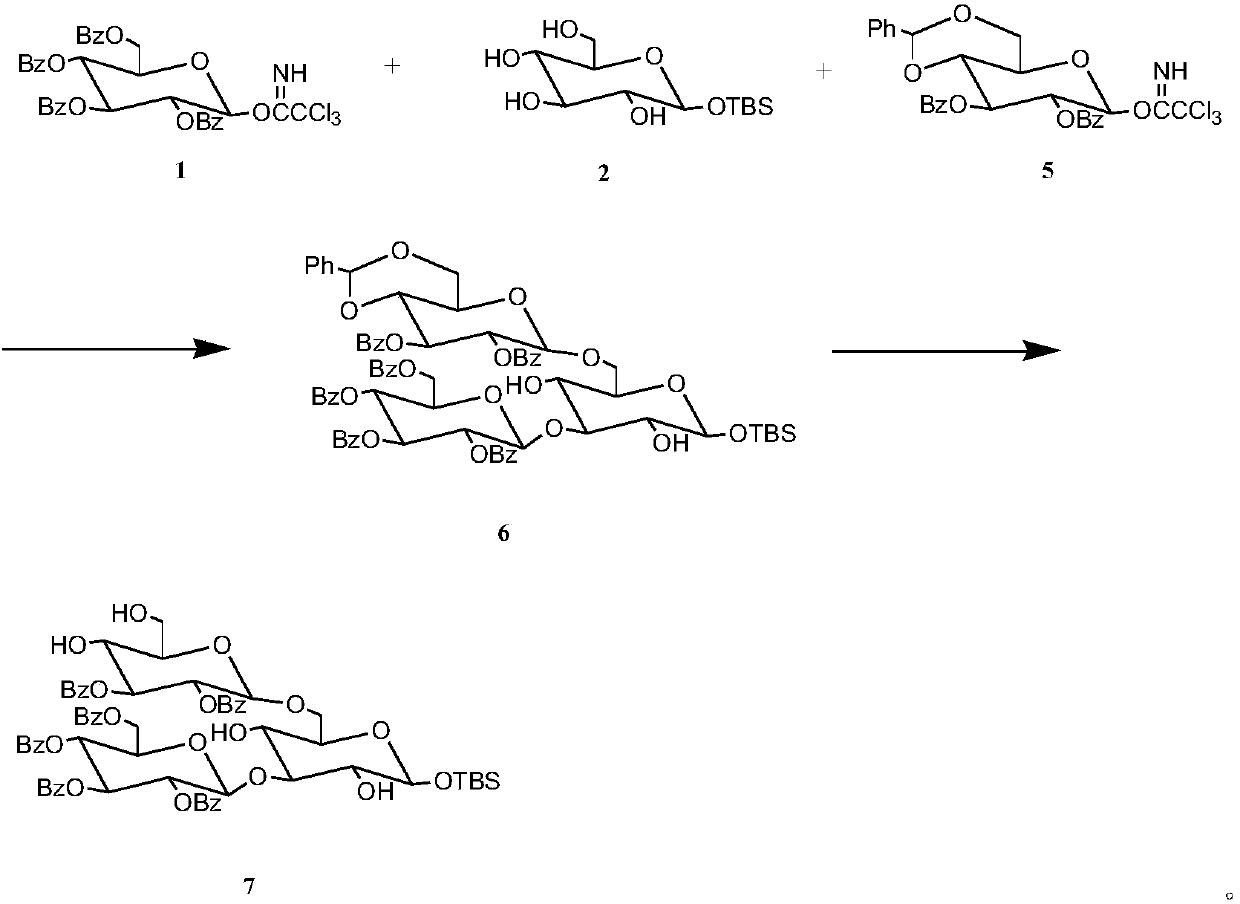
[0028] Preparation of Glucotriose Acceptor
[0029] 1) Dissolve 1.481g, 2mmol of benzoylglucose trichloroacetimidate in 10mL of dichloromethane to obtain solution a, and dissolve 0.588g, 2mmol of tert-butyldimethylsilyl-α-D-glucopyranose In 10mL of dichloromethane, to obtain solution b, 1.241g, 2mmol of 4,6-benzylidene-2,3-di-O-benzoylglucose trichloroacetimidate was dissolved in 10mL of dichloromethane, To obtain solution c, mix solution a, solution b, and solution c to obtain solution d, add 80 μ L, 0.70 mmol TMSOTf catalyst to solution d, add Molecular sieves, after reacting at 25°C for 3 hours, thin-layer chromatography analysis showed that the reaction was complete, filtered with suction, evaporated the solvent under reduced pressure, separated by column chromatography, and used ethyl acetate / cyclohexane (1 / 3) as eluent Rinse and collect the corresponding components to obtain pure trisaccharides with a yield of 89.7%.
[0030] 2) 1.330g, 1mmol trisaccharides were disso...
Embodiment 3
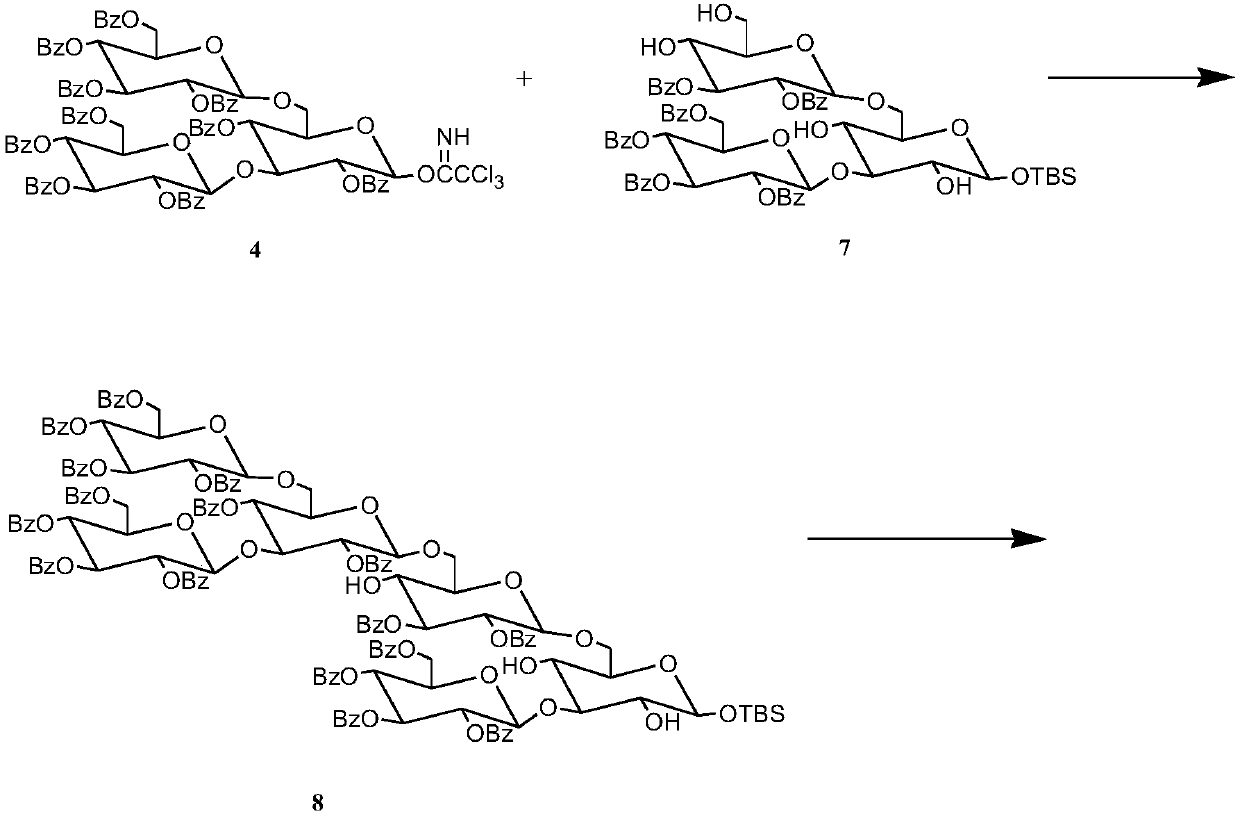
[0034] Synthesis of target compounds
[0035] 1) 3.377g, 2mmol triglucose donor, 2.484g, 2mmol triglucose acceptor and Molecular sieves were dissolved in 40mL of anhydrous dichloromethane, stirred for 50min under the protection of nitrogen, then 50μL, 0.44mmol TMSOTf catalyst was added dropwise, and reacted at 25°C for 4h. Thin-layer chromatography analysis showed that the reaction was complete, suction filtered, and evaporated under reduced pressure The solvent was separated by column chromatography, washed with ethyl acetate / cyclohexane (1 / 2) as eluent, and the corresponding components were collected to obtain pure hexasaccharide with a yield of 81.9%.
[0036] 2) Dissolve 5.536g, 2mmol hexasaccharide in 25mL, 1% hydrochloric acid-methanol solution, react at 25°C for 1-2h, thin-layer chromatography analysis shows that the reaction is complete, evaporate the solvent under reduced pressure, and separate by column chromatography, Ethyl acetate / cyclohexane (1 / 2) was used as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com