Method for determining activity of O-linked N-acetylglucosamine transferase and application of method
A technology of glucose transferase and acetylamino, which is applied in the field of biological analysis and detection, can solve the problems of inability to measure the activity of OGT, a large amount, etc., and achieves the effect of solving the problem of low catalytic activity and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
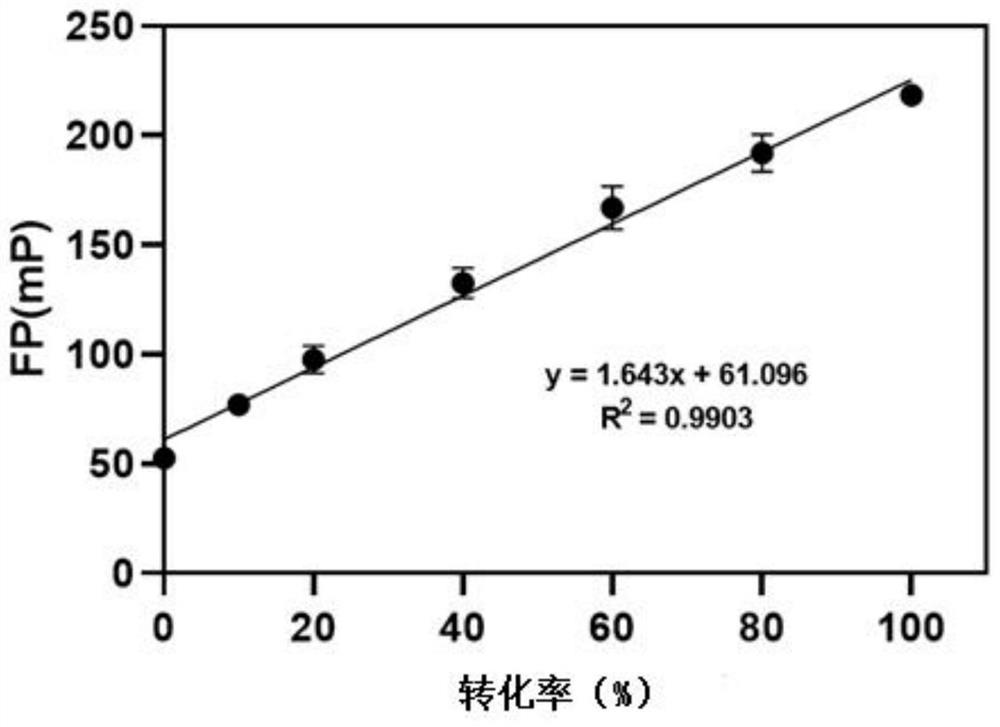
[0041] Example 1 Establishment of OGT activity assay method
[0042] Such as figure 2 As shown, the OGT activity assay method includes the following steps:
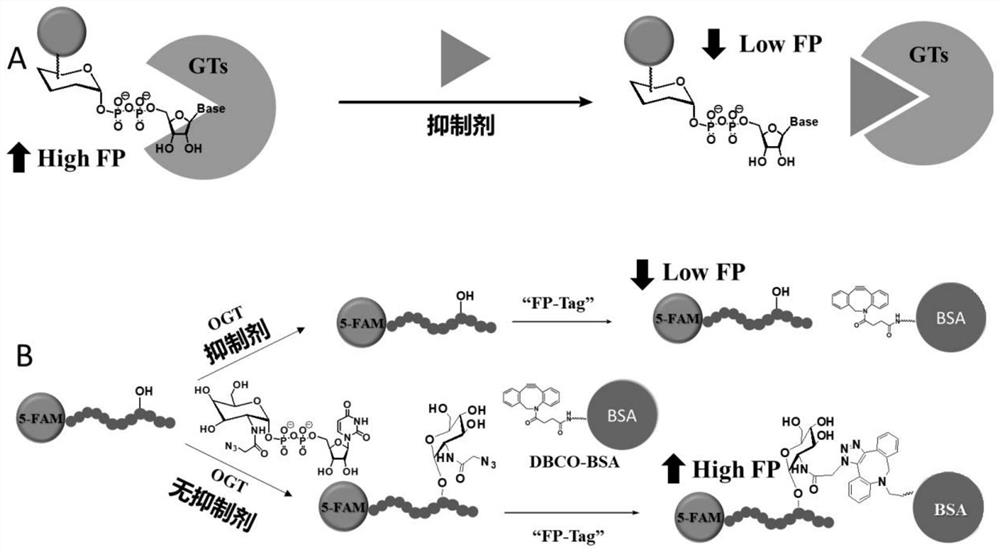
[0043] (1) Configure the OGT enzyme reaction system: containing 1 μM FAM-CKII peptide (its amino acid sequence is KKKYPGGSTPVSSANMM), 0.125mM UDP-GlcNAcN 3 , 50ng / μL ncOGT enzyme protein (no ncOGT enzyme protein was added to the control), buffer (150mM NaCl, 20mM Tris-HCl, 12.5mM MgCl 2 , 1 mM EDTA, pH 7.4).
[0044] (2) After incubating the above reaction system at 37°C in the dark for 6 hours, UDP with a final concentration of 1mM was added to terminate the reaction to obtain FAM-CKII-GlcNacN 3 .
[0045] (3) Mix bovine serum albumin (BSA) and dibenzocyclooctyne-active lipid-modified active lipid (DBCO-NHSester) at a molar ratio of 1:10, incubate at 37°C for 1 hour, and ultrafilter the unreacted DBCO-NHS ester is removed to generate DBCO-BSA complex;
[0046] (4) The reaction system (FAM-CKII-GlcNacN 3 ) was dil...
Embodiment 2
[0048] Example 2 Application of OGT activity assay method in high-throughput screening of OGT inhibitors
[0049] About 300 compounds in the marine natural product compound library preserved in the laboratory were screened for inhibitory activity. All reactions were carried out in a black 96-well plate (Cat.No.3694, Corning, USA). The specific screening method included the following steps:
[0050] (1) 10 mM natural product compound dissolved in DMSO (dimethyl sulfoxide) was diluted 40 times with 5% DMSO to obtain a sub-compound library.
[0051] (2) Add 30uL premix solution to the black 96-well plate, containing 50ng / μL ncOGT enzyme protein, 1μM FAM-CKII peptide (its amino acid sequence is KKKYPGGSTPVSSANMM), buffer (150mM NaCl, 20mM Tris-HCl, 12.5mMMgCl 2 , 1mM EDTA, pH 7.4), add 10μL of the compound of the sub-compound library (the compound of the sub-compound library in the OGT reaction system of the positive control), incubate at 37°C for 1 hour, then add 10uL of 0.125mM ...
Embodiment 3
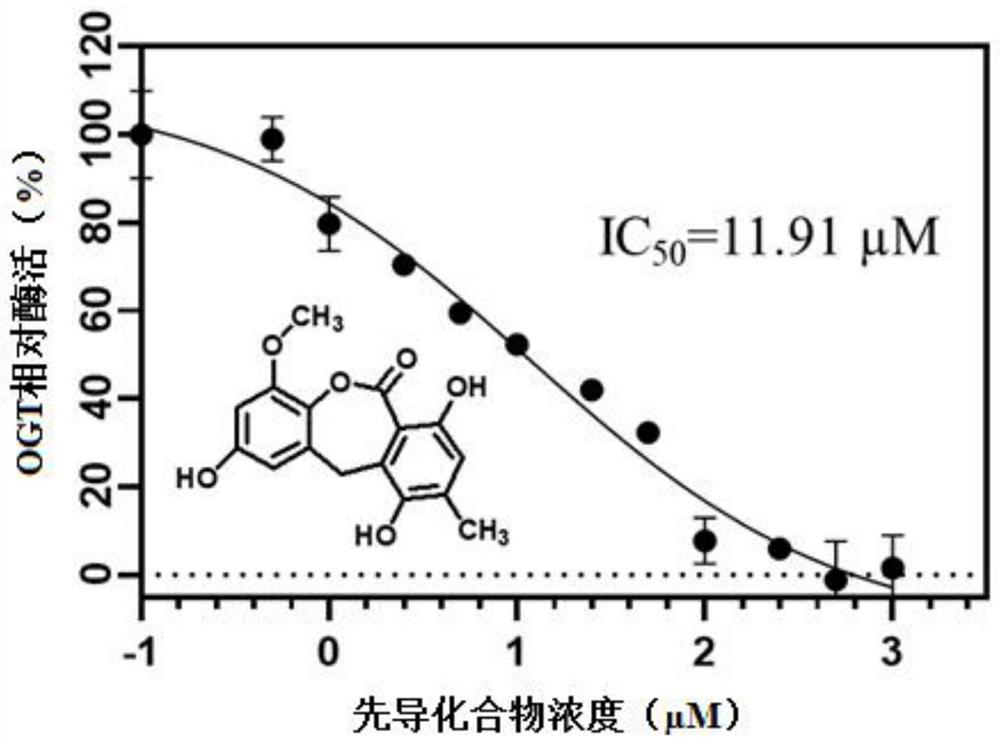
[0058] Example 3 Application of OGT Activity Determination Method in OGT Inhibitor Half Inhibitory Concentration Determination
[0059] Half-inhibitory concentration (IC 50 ) determination, the determination method may further comprise the steps:
[0060] (1) Dilute the lead compound to 0, 1, 2.5, 5, 10, 25, 50, 100, 250, 500, 1000, 2000 μM with 10% DMSO, respectively.
[0061] (2) Add 30uL premix solution to the black 96-well plate, containing 50ng / μL ncOGT enzyme protein, 1μM FAM-CKII peptide (its amino acid sequence is KKKYPGGSTPVSSANMM), buffer (150mM NaCl, 20mM Tris-HCl, 12.5mMMgCl 2 , 1mM EDTA, pH 7.4), add 10μL of lead compounds diluted to different concentrations, incubate at 37℃ for 1 hour, then add 10uL 0.125mM (final concentration) UDP-GlcNAcN 3 start reacting.
[0062] (3) After incubating the above reaction system at 37°C in the dark for 1 hour, UDP with a final concentration of 1 mM was added to terminate the reaction to obtain FAM-CKII-GlcNacN 3 .
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com