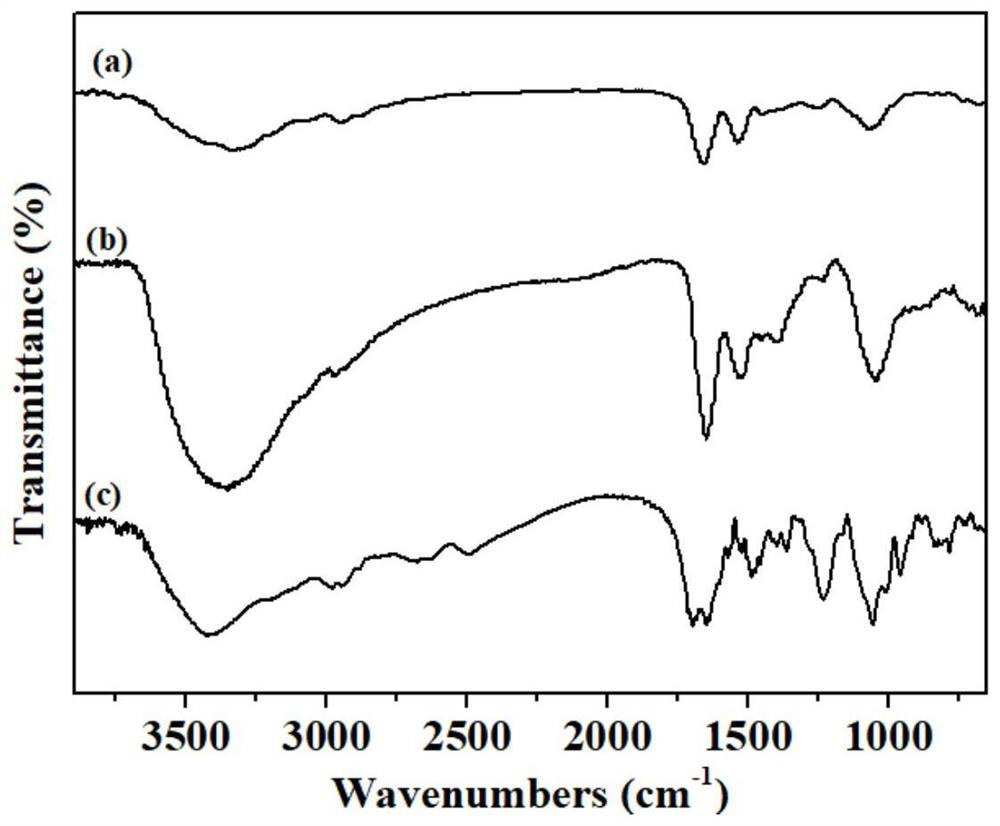
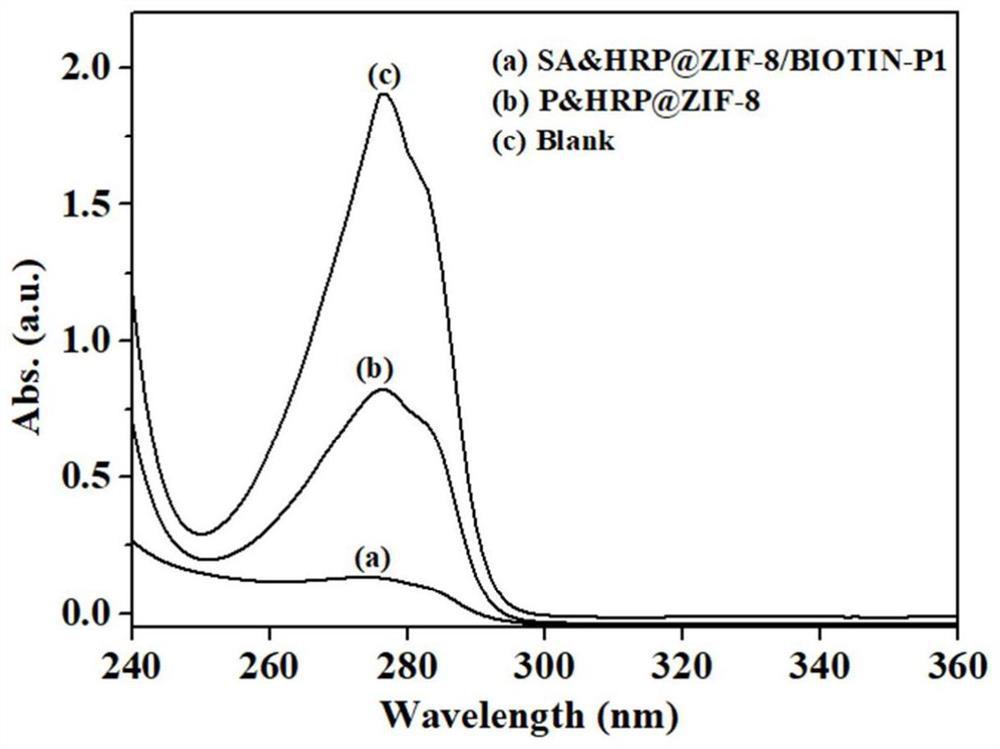
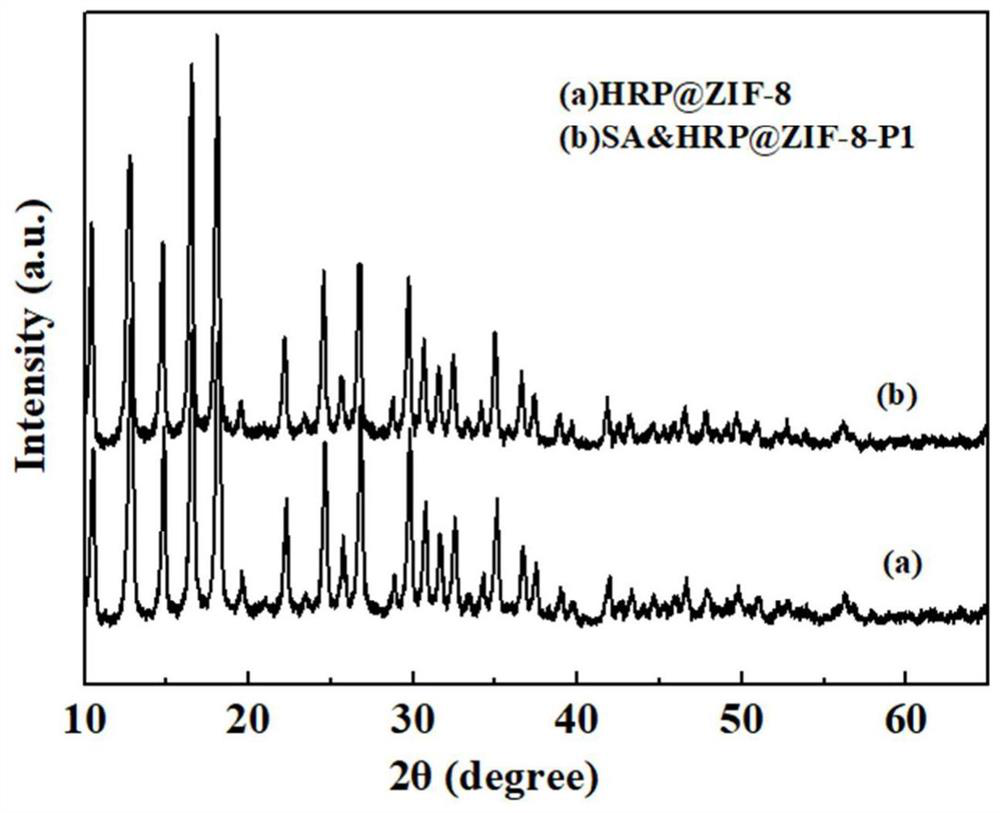
Preparation method and application of aptamer-functionalized horseradish peroxidase@metal-organic framework materials
A technology of transforming horseradish peroxide and organic skeleton, applied in biochemical equipment and methods, redox enzymes, chemical instruments and methods, etc., can solve problems such as difficult recycling, improve catalytic activity, and solve problems with low catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) SA&HRP@ZIF-8 / BIOTIN-P1 material preparation
[0044] Add SA solution (5mg / mL, 15μL) and HRP solution (2mg / mL, 60μL) to zinc nitrate hexahydrate solution (50mM, 250μL), mix the solution for 15min; then add 2-methylimidazole solution to the mixed solution (50mM, 250μL), mix well, put the mixture in a constant temperature water bath at 30°C and let it stand for 16h, centrifuge to collect the solid product and wash it with deionized water for 3 times; disperse the above obtained solid in P1 solution (100μM, 60μL) , centrifuged to separate the solid material, and washed three times with deionized water, and the final solid product was SA&HRP@ZIF-8 / BIOTIN-P1.
[0045] (2) P&HRP@ZIF-8 material preparation
[0046] Add HRP solution (2mg / mL, 60μL) and aptamer (P) solution (100μM, 60mL) to zinc nitrate hexahydrate solution (50mM, 250μL), mix and react for 15min; Imidazole solution (50mM, 250 μL), mixed well, put the mixed solution in a constant temperature water bath at 30°...
Embodiment 2
[0056] Selectivity experiment of SA&HRP@ZIF-8 / BIOTIN-P1
[0057] Bisphenol A (BPA), phenol and p-chlorophenol with a concentration of 0.07 mg / mL were prepared with NaAc-HAc (0.2M) buffer solution at pH 7.0, and an equal amount of SA&HRP@ZIF-8 was added to three solutions of the same volume / BIOTIN-P1 (100μL, 0.33mg / mL) and 100μL H 2 o 2 (0.3% w / w), after reacting at room temperature for 5 minutes, filter the reacted system with a filter membrane, and then measure the ultraviolet absorbance values of the obtained liquids at a wavelength of 277 nm. Such as Image 6 It was shown that under the same reaction conditions, both HRP@ZIF-8 and SA&HRP@ZIF-8 / BIOTIN-P1 had certain degradation performance on BPA, phenol and p-chlorophenol. Comparing the changes of absorbance values after the reaction of the three substrates, it was found that the degradation ability of SA&HRP@ZIF-8 / BIOTIN-P1 on BPA was significantly higher than that of phenol and p-chlorophenol.
Embodiment 3
[0059] Enzyme performance experiment of SA&HRP@ZIF-8 / BIOTIN-P1
[0060] Dilute the 20mg / mL BPA solution with pH7.0 NaAc-HAc (0.2M) buffer solution to a concentration of 0.29mM, 0.28mM, 0.26mM, 0.25mM, 0.23mM, take 500μL of the diluted BPA solution, and add 100μL SA&HRP@ZIF-8 / BIOTIN-P1(0.33mg / mL) and 100μL HO 2 o 2 (0.3%w / w) solution, make it fully react 5min, filter the mixed solution after the reaction with filter membrane, measure the ultraviolet absorbance value of gained liquid at 277nm place, determine the initial degradation rate of BPA by the ultraviolet absorbance of measuring solution, then Plot and fit kinetic curves; fit data using Michaelis-Menten equation to obtain V max and K m . The same experiment was performed with HRP@ZIF-8 in the control experiment. Such as Figure 7 As shown, in the control experiment, the K of HRP@ZIF-8 m The value is 18.2mM, while the K of SA&HRP@ZIF-8 / BIOTIN-P1 m The value is 6.7mM, indicating that aptamer P1 increases the effect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com