Synthetic method for 3,6-branched glucosepolyheptasaccharide
A synthesis method, glucose technology, applied in the field of synthesis of 3,6-position branched dextran heptasaccharide, can solve the problems of complex synthesis method of glucose oligosaccharides, unfavorable industrial production, low utilization rate of raw materials, etc., and achieve good self-defense immune system And the effect of regulating plant growth, high utilization rate of raw materials, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
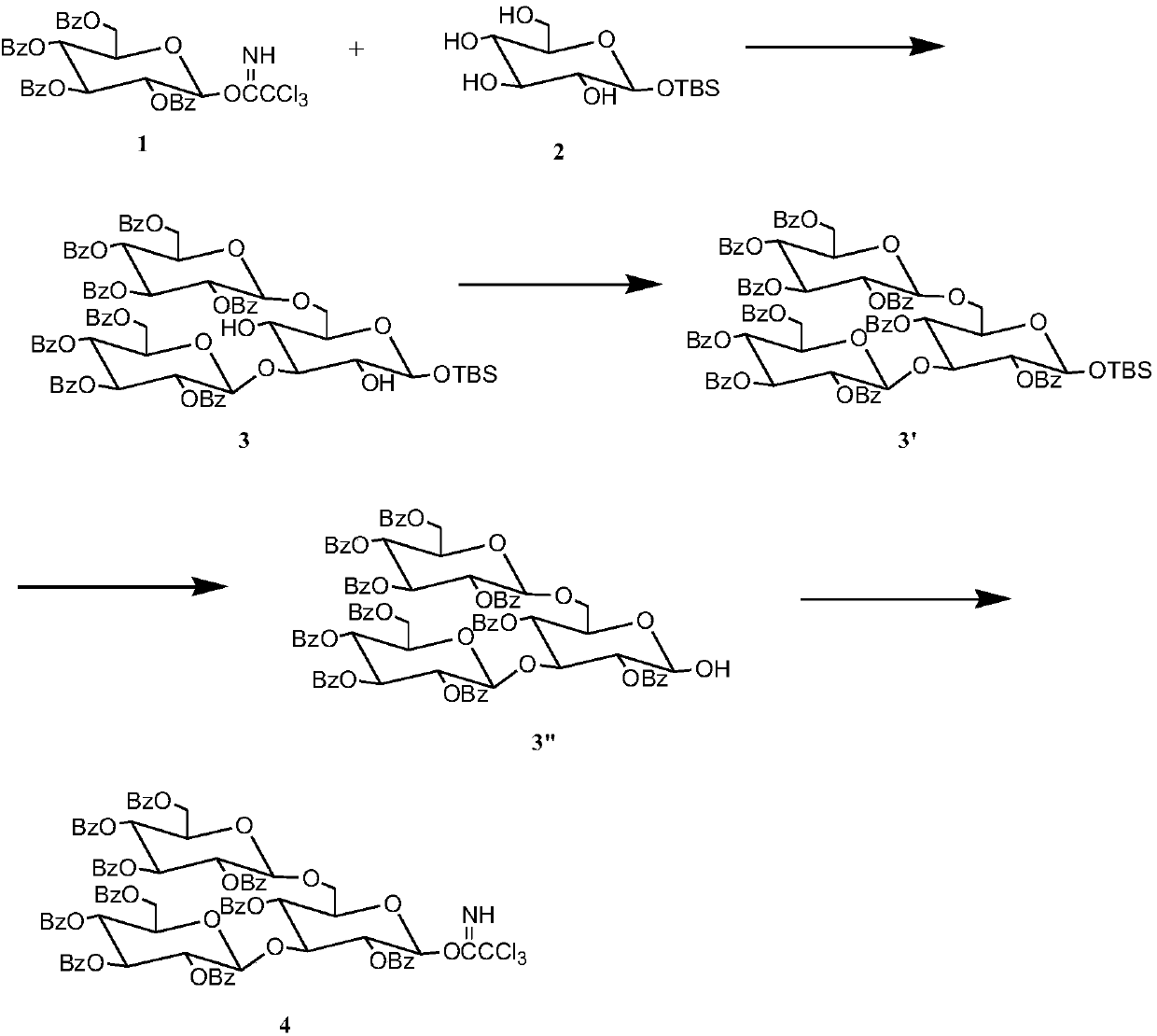
[0019] Preparation of glucotriose donor:
[0020] 1) Dissolve 3.705g, 5mmol of benzoylglucose trichloroacetimidate in 30mL of dichloromethane to obtain solution A, and dissolve 0.735g, 2.5mmol of tert-butyldimethylsilyl-α-D-glucopyranose Dissolve in 15mL of dichloromethane to obtain solution B, mix B and A to obtain solution C, add 230μL, 2mmol of TMSOTf catalyst to C, and then add Molecular sieves, after reacting at 25°C for 4 hours, thin-layer chromatography analysis showed that the reaction was complete, filtered with suction, evaporated the solvent under reduced pressure, separated by column chromatography, and used ethyl acetate / cyclohexane (1 / 3) as eluent Rinse and collect the corresponding components to obtain pure trisaccharides with a yield of 91.3%;
[0021] 2) Dissolve 7.250g, 5mmol trisaccharide in 30mL DMF, heat to 70°C to completely dissolve the glucose, add 2.9mL, 3.5mmol pyridine, take out the flask and cool it to 25°C, place the flask in an ice bath, add dro...
Embodiment 2
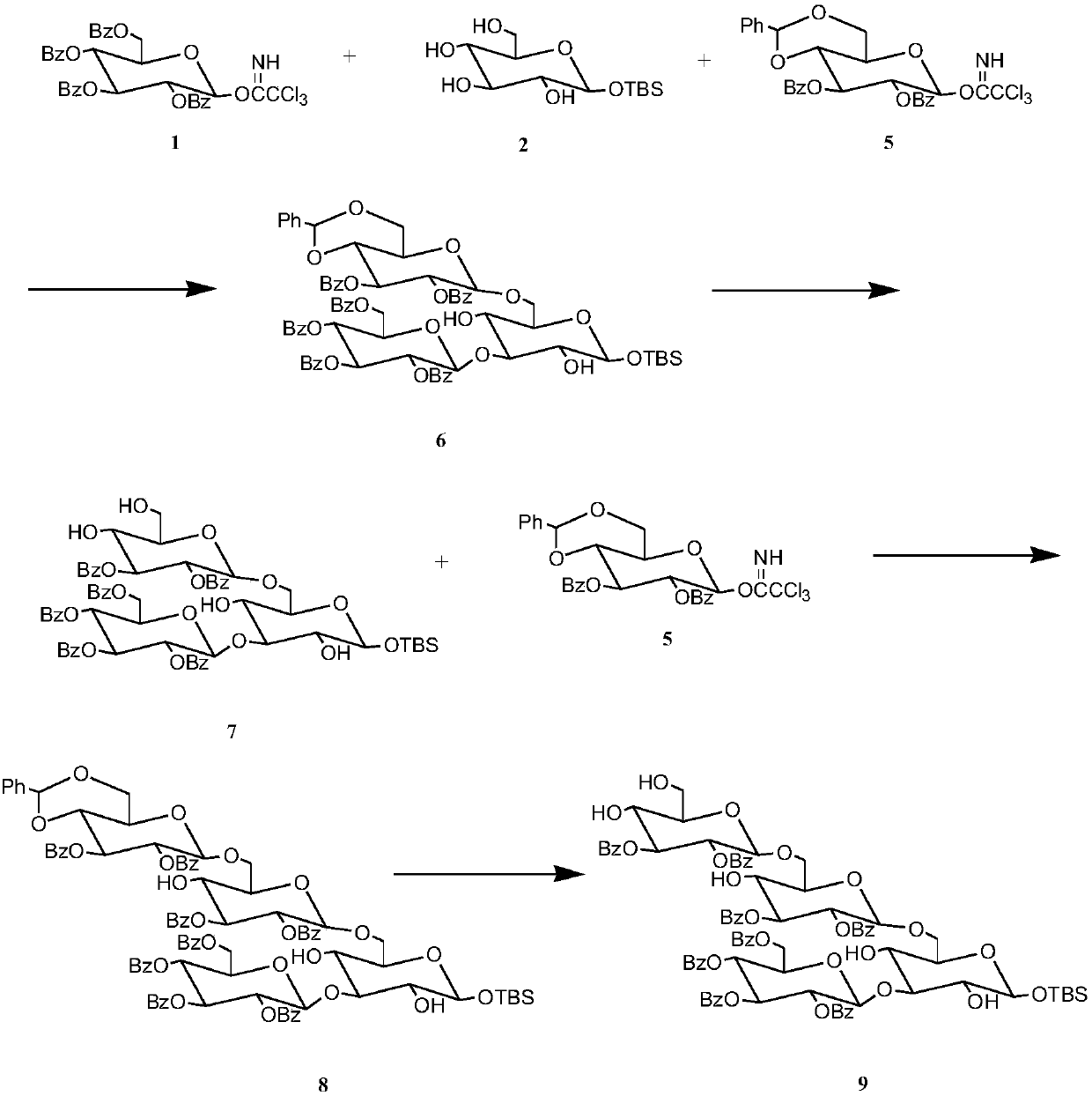
[0027] Preparation of glucotetraose acceptor
[0028] 1) Dissolve 1.481g, 2mmol of benzoylglucose trichloroacetimidate in 10mL of dichloromethane to obtain solution a, and dissolve 0.588g, 2mmol of tert-butyldimethylsilyl-α-D-glucopyranose In 10mL of dichloromethane, to obtain solution b, 1.241g, 2mmol of 4,6-benzylidene-2,3-di-O-benzoylglucose trichloroacetimidate was dissolved in 10mL of dichloromethane, To obtain solution c, mix solution a, solution b, and solution c to obtain solution d, add 80 μ L, 0.70 mmol TMSOTf catalyst to solution d, add Molecular sieves, after reacting at 25°C for 3 hours, thin-layer chromatography analysis showed that the reaction was complete, filtered with suction, evaporated the solvent under reduced pressure, separated by column chromatography, and used ethyl acetate / cyclohexane (1 / 3) as eluent Rinse and collect the corresponding components to obtain pure trisaccharides with a yield of 89.7%.
[0029] 2) 1.330g, 1mmol trisaccharides were dis...
Embodiment 3
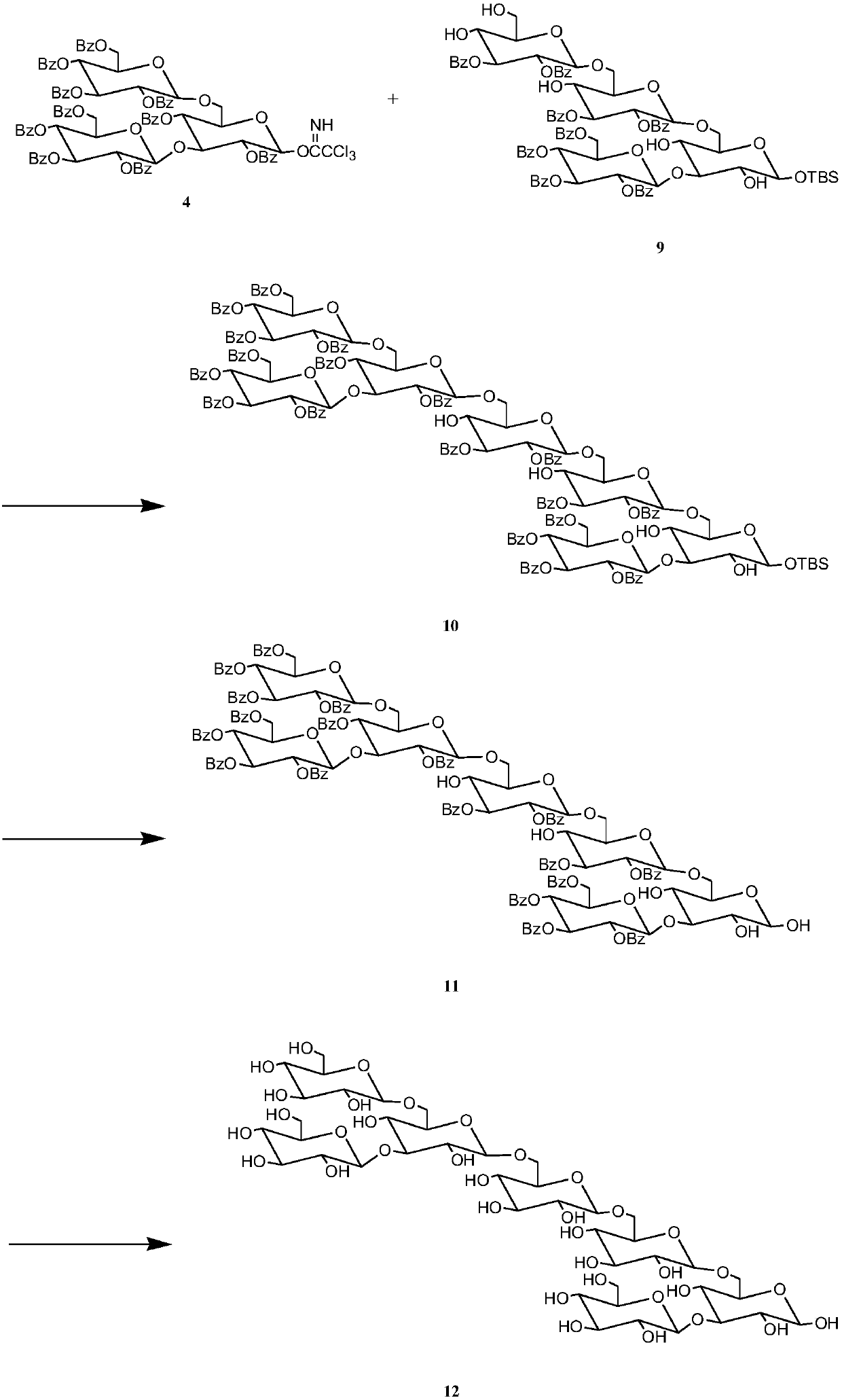
[0035] Synthesis of target compounds
[0036] 1) 3.377g, 2mmol glucotriose donor, 3.224g, 2mmol glucotetraose acceptor and Molecular sieves were dissolved in 50 mL of anhydrous dichloromethane, stirred under nitrogen protection for 1.5 h, then 50 μL, 0.44 mmol of TMSOTf catalyst was added dropwise, and reacted at 25 °C for 4.5 h, thin-layer chromatography analysis showed that the reaction was complete, suction filtered, and reduced pressure The solvent was evaporated, separated by column chromatography, washed with ethyl acetate / cyclohexane (1 / 1.8) as eluent, and the corresponding components were collected to obtain pure heptasaccharide with a yield of 79.6%.
[0037]2) Dissolve 6.276g, 2mmol heptasaccharide in 35mL, 1% hydrochloric acid-methanol solution, react at 25°C for 2-2.5h, thin-layer chromatography analysis shows that the reaction is complete, evaporate the solvent under reduced pressure, and separate by column chromatography , using ethyl acetate / cyclohexane (1 / 1.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com