Azole derivative, method for producing same, intermediate compound, and agricultural or horticultural chemical agent and industrial material protecting agent
A technology of azole derivatives and production method, applied in the field of azole derivatives, can solve problems such as no specific disclosed compounds, and achieve the effect of excellent bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
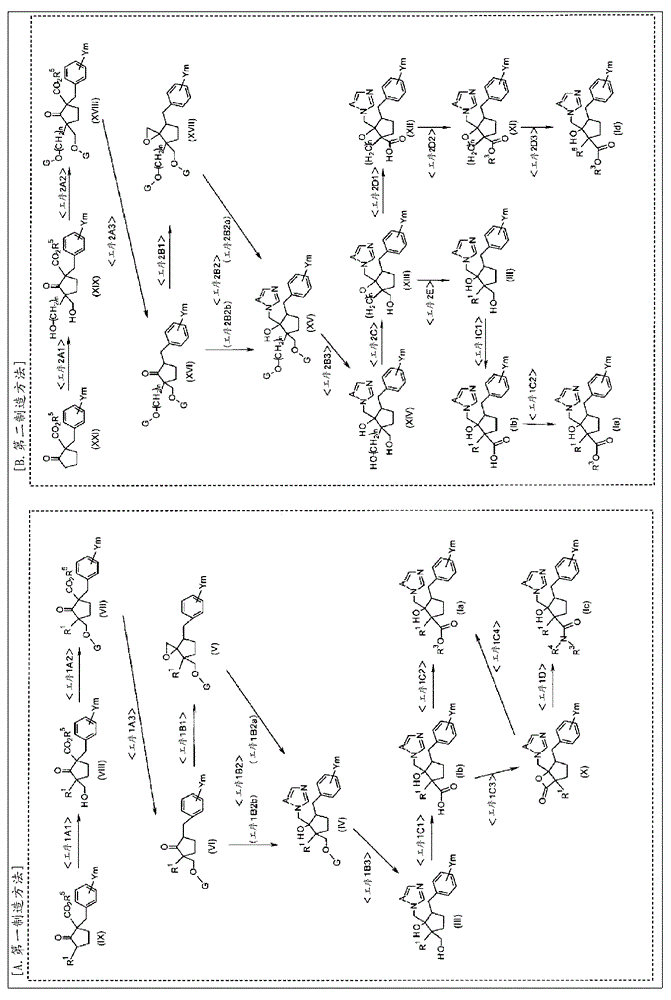
preparation example Construction
[0284] Metal alkoxides can be prepared by making alcohols (R 3 OH) is obtained by reacting with alkyllithium, metal sodium, metal lithium or sodium hydride in solvents such as THF and diethyl ether. Among them, a method of reacting with metal lithium is preferably used, and a method of reacting with alkyllithium is more preferably used.
[0285] The amount of the metal alkoxide is usually 0.5-fold mol to 20-fold mol, preferably 0.8-fold mol to 10-fold mol, based on the lactone compound (X).
[0286] As a solvent, THF, diethyl ether, dioxane or the like can be used, and it is preferable to use the same solvent as that used for the preparation of the metal alkoxide.
[0287] The reaction temperature and reaction time are appropriately set and used according to the reagents used. The reaction temperature is preferably -100°C to 200°C, more preferably -80°C to 150°C. In addition, the reaction time is preferably 0.1 hour to several days, more preferably 0.5 hour to 2 days.
[0...
manufacture example 1
[0448] (1SR,2SR,3RS)-3-(4-chlorobenzyl)-2-hydroxy-1-methyl-2-(1H-1,2,4-triazol-1-ylmethyl)-1- Cyclopentane carboxylic acid (compound (I-1): R 1 =CH 3 , R 2 =COOH, A=N, Ym=4-Cl, stereo configuration CC) synthesis
[0449] 6.03 g of chromic acid was dissolved in 11.3 ml of water, and 5.2 ml of concentrated sulfuric acid was slowly added dropwise. 1.8 ml of water was added to the salt generated here to dissolve it, and Jones reagent was prepared. (1RS, 2SR, 3RS)-3-(4-chlorobenzyl)-2-hydroxy-1-methyl-2-(1H- 1,2,4-triazol-1-ylmethyl)-1-cyclopentanemethanol (compound (III-1):R 1 =CH 3 , A=N, Ym=4-Cl, stereo configuration CC) 1.44 g was dissolved in 45 ml of acetone, 3.3 ml of the previously prepared Jones reagent was added thereto, and stirred at room temperature for 1.5 hours.
[0450] After the reaction was completed, isopropanol was added, the green insoluble matter was filtered off, and then washed with acetone, the filtrate and the washing liquid were combined, the combi...
manufacture example 2
[0453] (1RS,2SR,3RS)-3-(4-chlorobenzyl)-2-hydroxy-1-methyl-2-(1H-1,2,4-triazol-1-ylmethyl)-1- Cyclopentane carboxylic acid (compound (I-131): R 1 =CH 3 , R 2 =COOH, A=N, Ym=4-Cl, stereo configuration TC)
[0454] Instead of using (1SR,2SR,3RS)-3-(4-chlorobenzyl)-2-hydroxy-1-methyl-2-(1H-1,2,4-triazol-1-ylmethyl)- 1-cyclopentanemethanol (compound number (III-2): R 1 =CH 3 , A=N, Ym=4-Cl, stereo configuration TC) to replace (1RS, 2SR, 3RS)-3-(4-chlorobenzyl)-2-hydroxyl-1-methyl-2-(1H- 1,2,4-Triazol-1-ylmethyl)-1-cyclopentanemethanol (compound number (III-1)) was used as a raw material, by the same method as the method for obtaining the above-mentioned compound (I-1) to obtain the target compound.
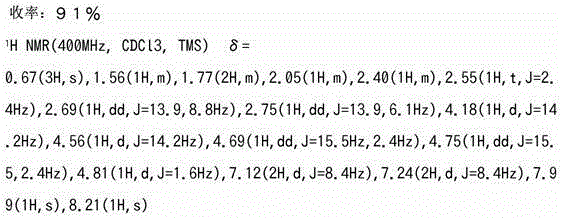
[0455]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com