Batch synthesis method for 4-O-beta-Galactopyranosyl-D-mannopyranoside
A synthesis method and batch technology are applied in the field of batch synthesis of epilactose, and can solve the problems of heavy chemical synthesis workload and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
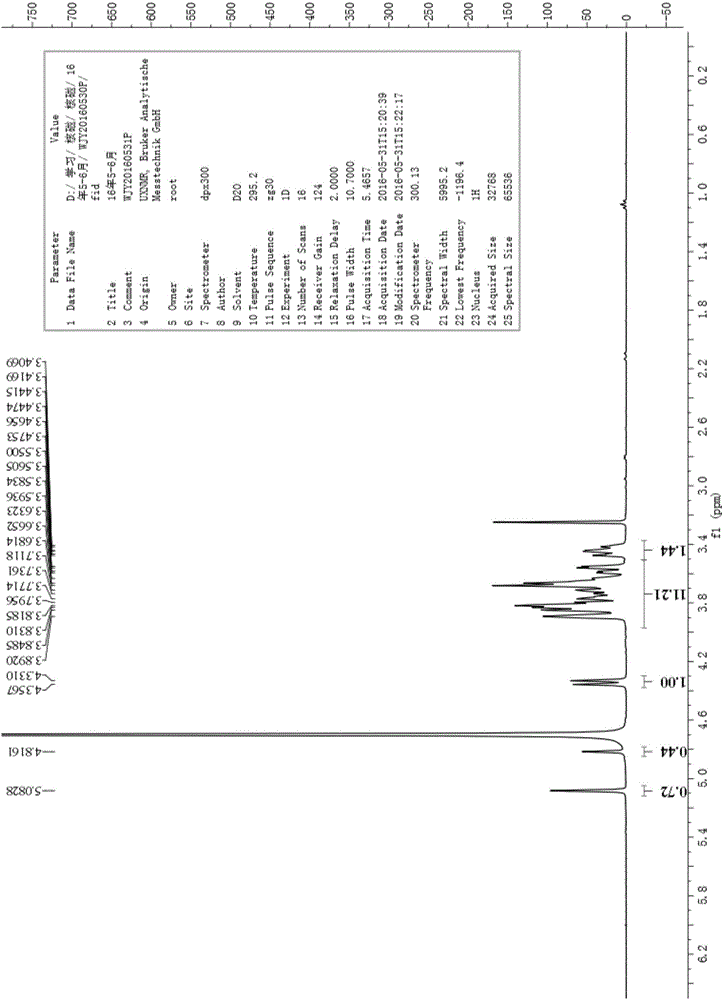
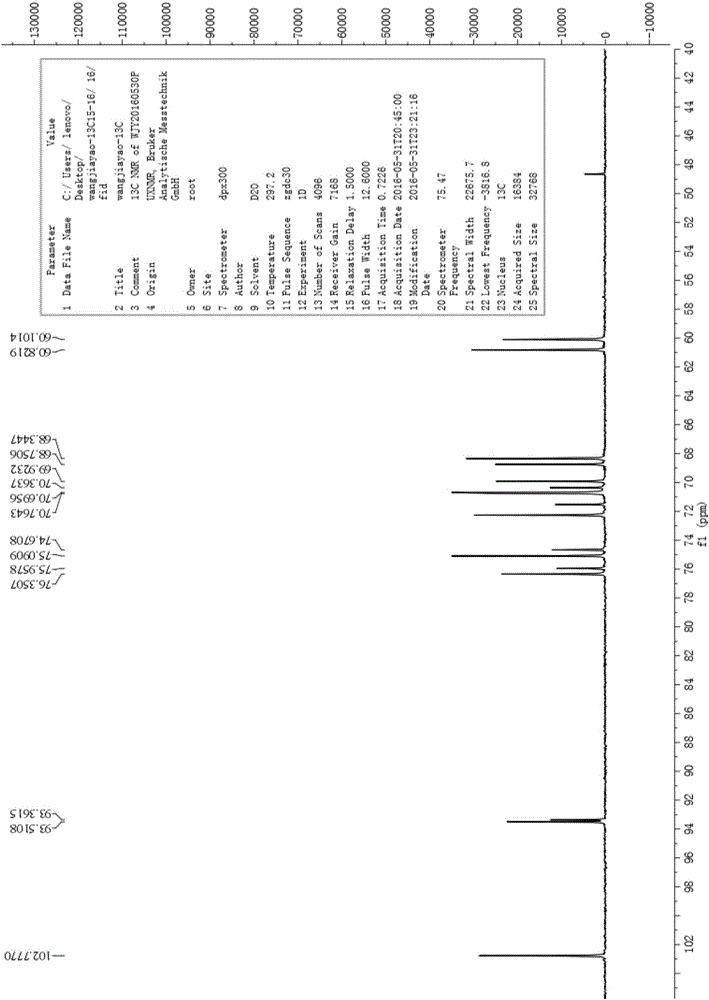
[0101] Embodiment 1, batch synthesis epilactose
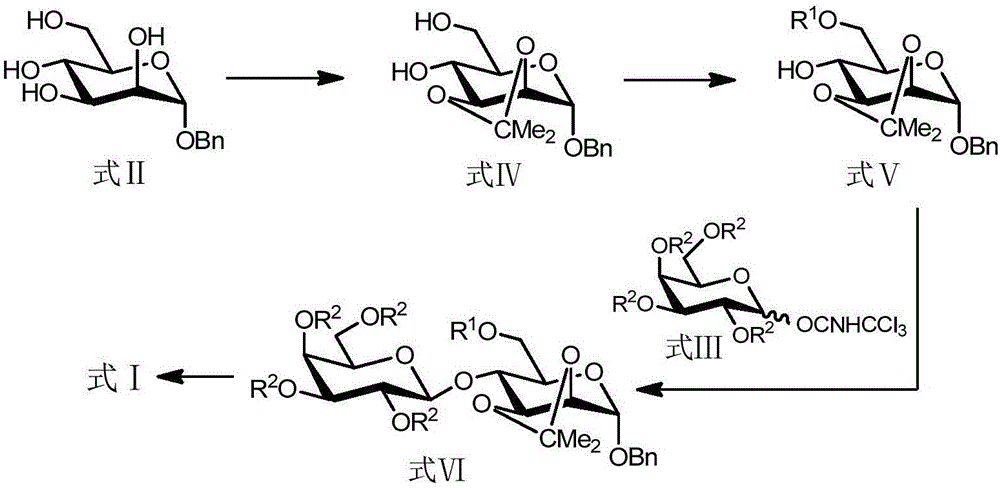
[0102] according to figure 1 The synthetic route shown is the batch synthesis of epilactose, and the specific steps are as follows:
[0103] (1) Synthesis of compound shown in formula IV:
[0104]
[0105] Compound II (20 g, 0.074 mol), p-toluenesulfonic acid (7 g, 0.037 mol) and magnetons were added into a 200 ml round bottom flask. Under nitrogen protection, 100ml of dry DMF and 2-methoxypropene (7.27ml, 0.078mol) were added and reacted at room temperature (25°C) for 2 hours. TLC [V (petroleum ether) / V (ethyl acetate) = 1:2] detected that the reaction was complete, and placed it in an oil bath at 70° C. to react overnight (ie, 12 hours). TLC [V (petroleum ether) / V (ethyl acetate) = 1:2] detected that the reaction was complete, and 10 ml of triethylamine was added to terminate the reaction. DMF was distilled off under reduced pressure with 30 mL×3 toluene to obtain a crude product. Recrystallization with V (petroleum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com