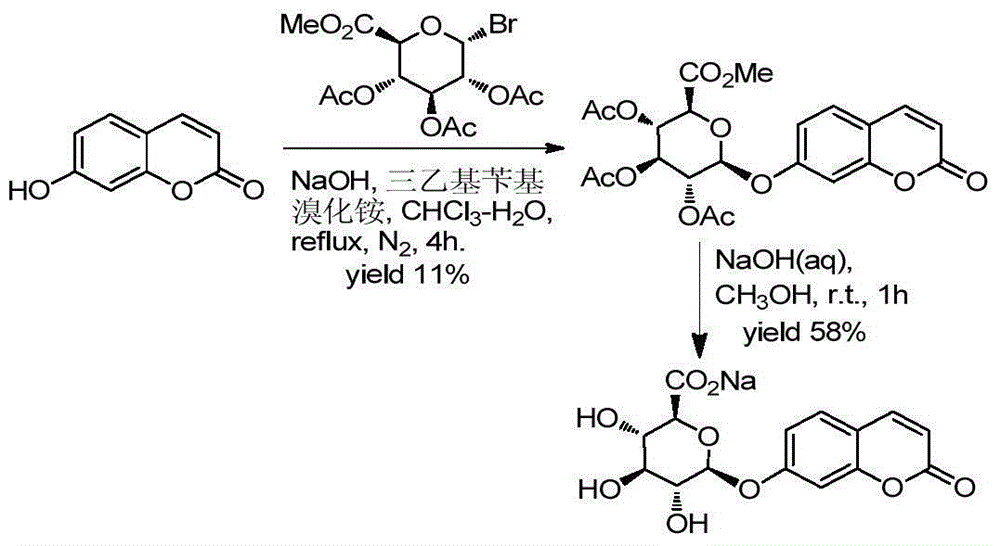
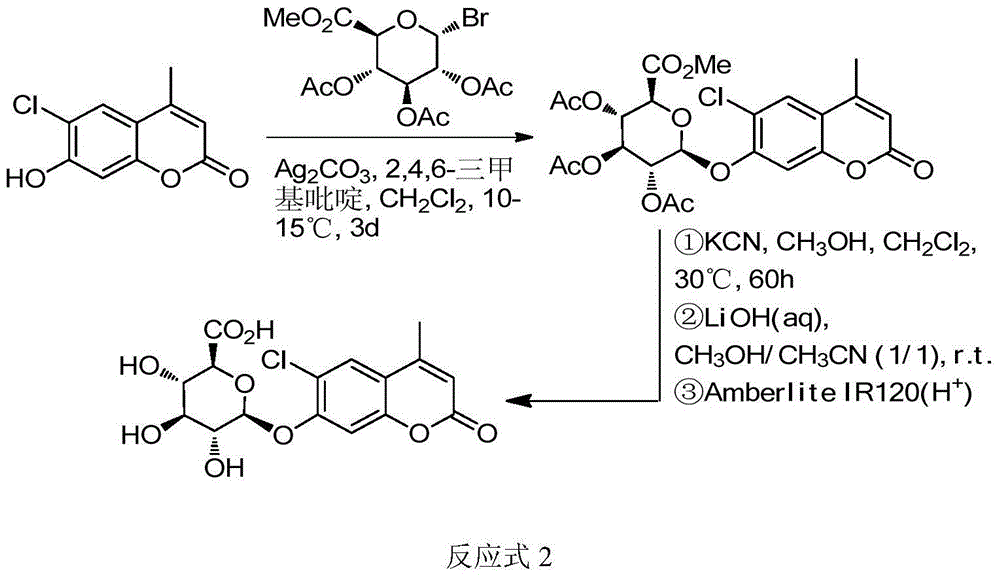
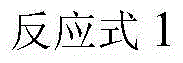Method for synthesizing various glucosides on basis of 4-methylumbelliferone
A kind of technology of methyl umbelliferone and glycoside, applied in the field of chemical synthesis, can solve the problems of long reaction time, poor stereoselectivity, difficult source and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] To the 4-MU (0.264g, 1.50mmol, 1.0e.q.) and 1,2,3,4-tetra-O-acetyl-β-D-glucopyranose methyl ester (0.376g , 1.00mmol, 0.67e.q.) of dry CH 2 Cl 2 (3ml) suspension, add dry Et 3 N (525μL, 3.75mmol, 2.5e.q.), BF 3 .OEt 2 (1287μL, 10.00mmol, 6.7e.q.), stirred the reaction at room temperature for 72h, then added 3ml CH 2 Cl 2 diluted with Na 2 CO 3 solution or saturated NaHCO 3 solution to stop the reaction, then wash with dilute NaOH solution until the solution becomes light or alkaline, then wash with water and saturated saline in sequence, and wash with anhydrous NaOH after separation. 2 SO 4 The organic phase was dried, and the separated organic phase was concentrated, followed by flash column chromatography (200-300 mesh silica gel, eluted with petroleum ether II / ethyl acetate=5 / 2), and the obtained target separation liquid After removing the solvent by rotary evaporation, it was recrystallized with anhydrous ether and dried to obtain 0.084 g of white powder (...
Embodiment 2
[0044] To the 4-MU (0.264g, 1.50mmol, 1.0e.q.) and 1,2,3,4-tetra-O-acetyl-β-D-glucopyranose methyl ester (0.376g , 1.00mmol, 0.67e.q.) of dry ClCH 2 CH 2 To the Cl (3ml) suspension, add dry Et 3 N (525μL, 3.75mmol, 2.5e.q.), BF 3 .OEt 2 (1287μL, 10.00mmol, 6.7e.q.), stirred the reaction at 60°C for 5h, then added 3ml CH 2 Cl 2 diluted with Na 2 CO 3 solution or saturated NaHCO 3 solution to stop the reaction, then wash with dilute NaOH solution until the solution becomes light or alkaline, then wash with water and saturated saline in sequence, and wash with anhydrous NaOH after separation. 2 SO 4 The organic phase was dried, and the separated organic phase was concentrated, followed by flash column chromatography (200-300 mesh silica gel, eluted with petroleum ether II / ethyl acetate=5 / 2), and the obtained target separation liquid After removing the solvent by rotary evaporation, it was recrystallized with anhydrous ether and dried to obtain 0.082 g of white powder (1...
Embodiment 3
[0046] To the 4-MU (0.352g, 2.00mmol, 1.0e.q.) and 1,2,3,4-tetra-O-acetyl-β-D-glucopyranose methyl ester (0.376g , 1.00mmol, 0.50e.q.) of dry ClCH 2 CH 2 To the Cl (3ml) suspension, add dry Et 3 N (280μL, 2.00mmol, 1.0e.q.), BF 3 .OEt 2 (1287μL, 10.00mmol, 6.7e.q.), stirred the reaction under heating and reflux for 5h, then added 3ml CH 2 Cl 2 diluted with Na 2 CO 3 solution or saturated NaHCO 3 solution to stop the reaction, then wash with dilute NaOH solution until the solution becomes light or alkaline, then wash with water and saturated saline in sequence, and wash with anhydrous NaOH after separation. 2 SO 4 The organic phase was dried, and the separated organic phase was concentrated, followed by flash column chromatography (200-300 mesh silica gel, eluted with petroleum ether II / ethyl acetate=5 / 2), and the obtained target separation liquid After removing the solvent by rotary evaporation, it was recrystallized with anhydrous ether and dried to obtain 0.088 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com