Glycosylation reaction catalyst, glycosylation method and application
A glycosidation reaction and catalyst technology, which is applied in the field of sugar chemistry, can solve the problems of lack of universal synthetic methods with wide applicability, and achieve the effects of accelerated reaction rate, high practical value, and excellent reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
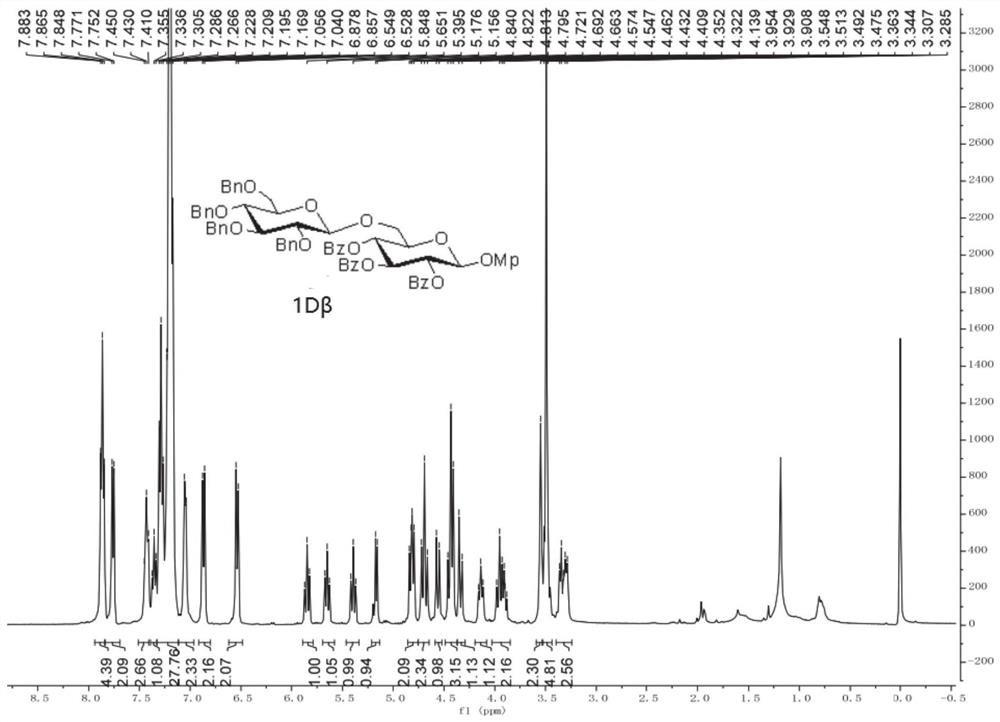
[0066] 1. 1A-1H, 2A, 3C, 4C, 4E, 5C, and 5F are prepared according to the following operating procedures in this embodiment 1:
[0067]
[0068] The glycosylation reaction of the acceptor as a monohydroxy compound was carried out using the standard operating procedure (1): under an inert gas environment, the acceptor (1.2 equivalents) was added to the dissolved glycosyl donor (1.0 equivalents) and dry MS (100 mg / ml) in anhydrous DCM (0.01 M) was stirred in the greenhouse. The reaction mixture was then cooled to -65°C, and platinum(IV) chloride (0,1 equiv) was added thereto. Upon completion by TLC plate, the reaction was quenched by adding Et3N, diluted with DCM, and the precipitate was filtered off through a pad of Celite. The organic layer was washed with NaHCO3 (aq.) and NaCl (aq.), dried over Na2SO4, filtered and the solvent was removed under reduced pressure distillation. The resulting residue was purified by silica gel column chromatography to obtain the correspondi...
Embodiment 2
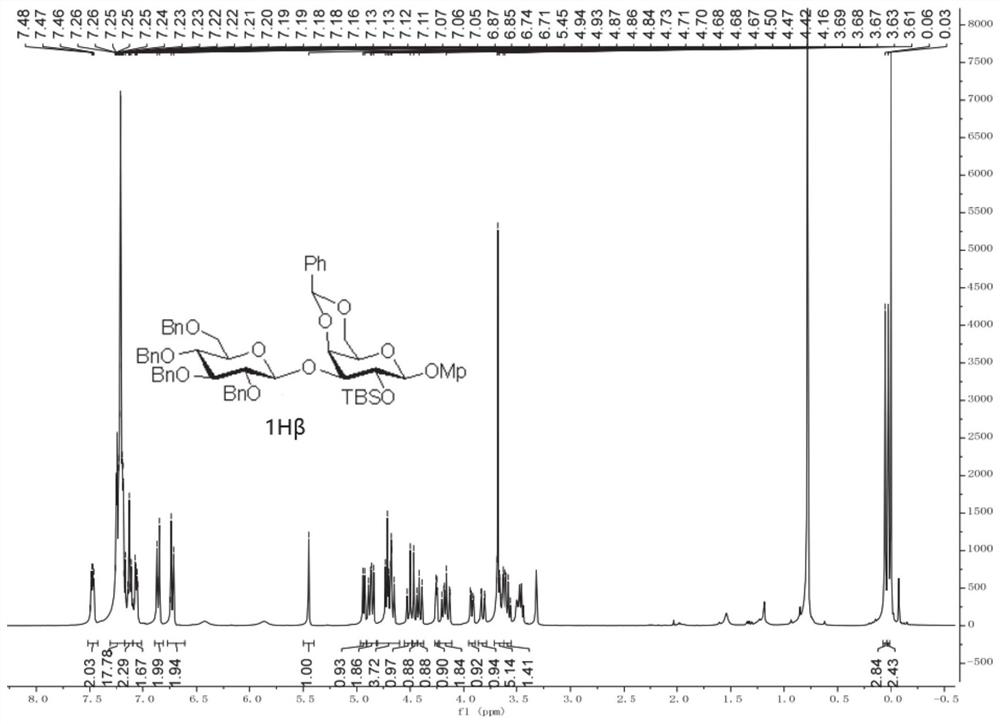
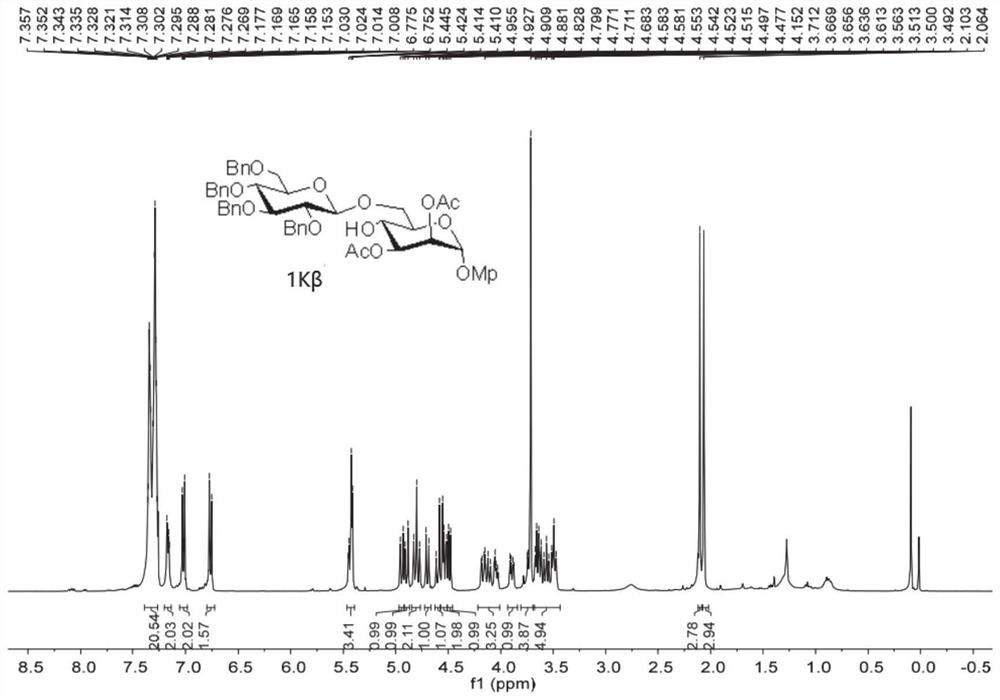
[0075] Embodiment 2: In this embodiment 2, 1I-1K, 1L-1P are prepared according to the following operating procedure:
[0076]
[0077] The acceptor is the glycosylation reaction of 1,2-cis-diol and 1,3-diol compound, and the standard operating procedure (2) is used: under an inert gas environment, the acceptor (1.2 equivalents) is added to the dissolved Glycosyl donor (1.0 equiv) and dry MS (100 mg / ml) in anhydrous DCM (0.01 M) was stirred in the greenhouse. The reaction mixture was then cooled to -78°C, and platinum(IV) chloride (0,1 equiv) was added thereto. It was detected by TLC plate until the reaction was completed, and finally Et was added 3 The reaction was quenched with N, diluted with DCM, and the precipitate was filtered through a pad of celite. NaHCO for organic layer 3 (aq.) and NaCl (aq.), washed with NaCl 2 SO 4 Dry, filter and remove the solvent under reduced pressure distillation. The resulting residue was purified by silica gel column chromatograph...
Embodiment 3
[0083] Embodiment 3: In this embodiment 3, 1Q-1Z is prepared according to the following operating procedures:
[0084]
[0085] The acceptor is the glycosylation reaction of 1,2-trans-diol and 1,2,3-triol, which is carried out by using the reaction standard operating procedure (3): under an inert gas environment, dissolve the acceptor (1.2 equivalents) into with dry MS (100 mg / ml) in anhydrous DCM (0.01 M) with stirring in the greenhouse. The reaction mixture was then cooled to -78°C, platinum(IV) chloride (0,1 eq) was added thereto and stirred for 10 min, and finally the glycosyl donor (1,0 eq) dissolved in anhydrous DCM was slowly added to the reaction middle. Upon completion by TLC plate, the reaction was quenched by adding Et3N, diluted with DCM, and the precipitate was filtered off through a pad of Celite. NaHCO for organic layer 3 (aq.) and NaCl (aq.), washed with NaCl 2 SO 4 Dry, filter and remove the solvent under reduced pressure distillation. The resulting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com