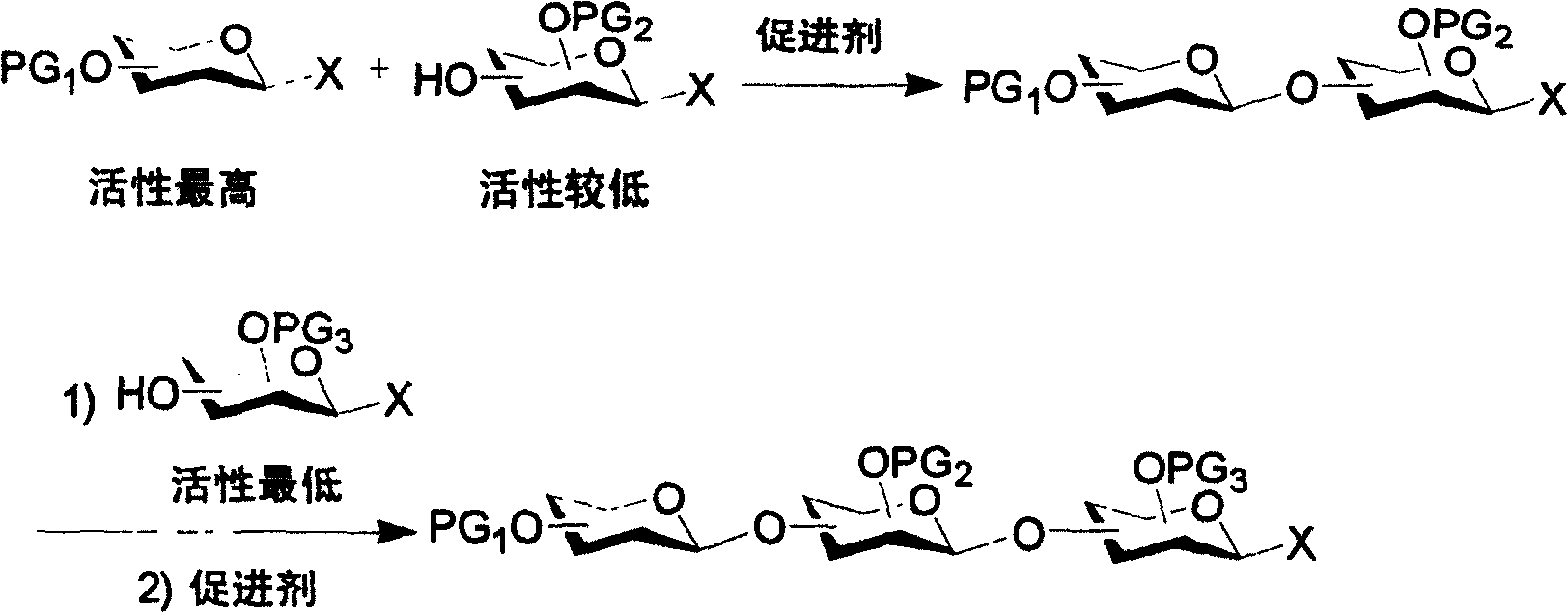
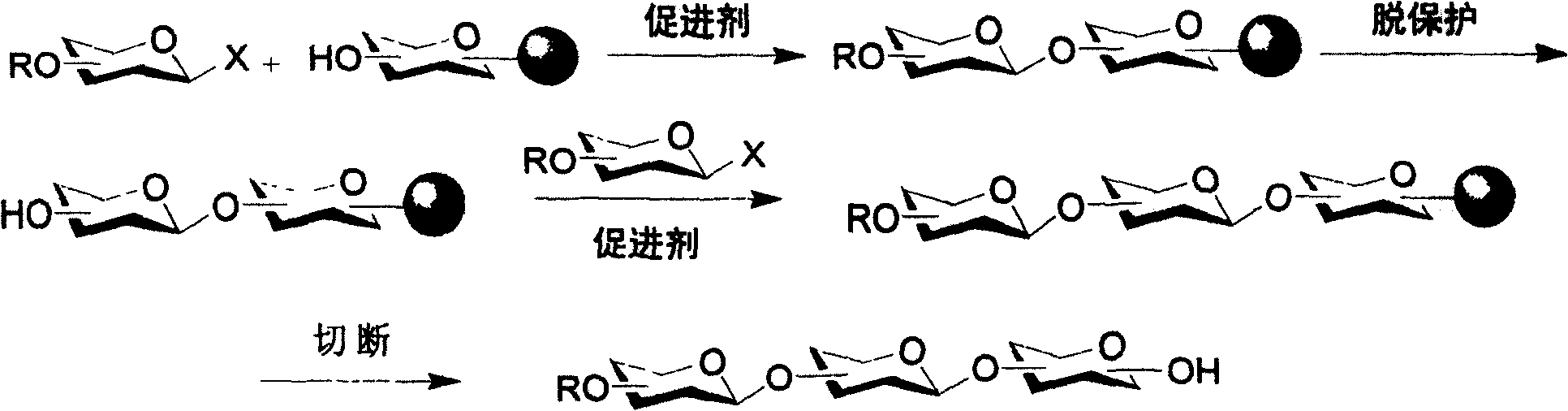
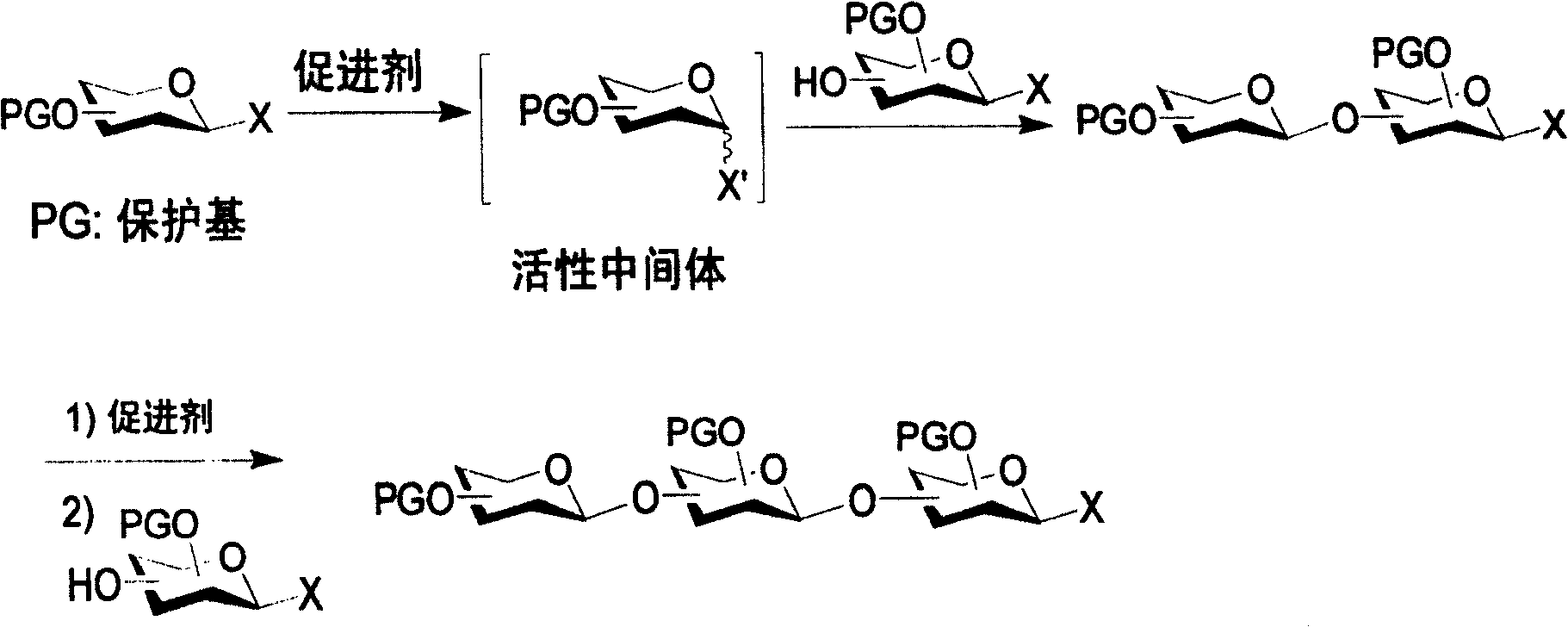
Iterative oligosaccharide synthesis
A synthesis method and oligosaccharide technology, applied in the field of sugar chemistry, can solve the problems of unforeseen reaction process monitoring, hindering the development and difficulties of oligosaccharide solid-phase synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] At a temperature of -60°C, add 1 equivalent of p-toluenesulfur chloride (TolSCl) to the ether solution of active donor 1 and silver trifluoromethanesulfonate (AgOTf), and use AW300 molecular sieves as a dewatering agent. After the donor 1 is fully activated, the ether solution of the active donor / acceptor 4 is added, and the disaccharide 2 is obtained in a yield of 74%. The ratio of α and β isomers is 3.3:1 (Table 1, No. 1 ), the anomeric position of disaccharide 2 has a p-tolylthio group. Disaccharide 2 was glycosylated with donor / acceptor 4 to obtain trisaccharide 9 with a yield of 71% (No. 2). The hydroxyl group on the vertical bond of the active donor / acceptor 5 reacted with the donors 1 and 3 to form disaccharides 10 and 11, respectively, with a yield of 66% (No. 3 and 4).
[0068] p-Tolylthiotriflate (TolSOTf) is a strong activator capable of stoichiometrically activating an inactive donor. The inactive donor 6 can successfully react with the very active donor / a...
Embodiment 2
[0074] Figure 3. One-pot oligosaccharide synthesis
[0075]
[0076] To test the feasibility of an activity-independent one-pot method for multi-step glycosylation reactions, we synthesized several oligosaccharides by sequentially adding different building blocks in the same reaction vessel9,16,17 and 18 (Fig. 3). It is worth noting that the yield of trisaccharide 9 synthesized by the one-pot method is 66% (Fig. 3a), while the total yield of the stepwise synthesis is only 53% (Table 1, No. 1, 2). We used different structural modules 6, 4, 7, 19 to synthesize tetrasaccharide 16 by one-pot method, used structural modules 3, 5, 7, 20 to obtain tetrasaccharide 17, and used structural modules Sugar 18, the total yields were 68%, 55% and 48%, respectively. Therefore, the one-pot synthesis method not only saves the separation and purification of intermediates, but also improves the overall yield of the reaction through the reduction of purification steps. The final oligosacchar...
Embodiment 3
[0078] Figure 4
[0079]
[0080] In another example of the present invention, an affinity tag is introduced into the oligosaccharide to simplify the purification of the target oligosaccharide product (Figure 4).
[0081] The introduction of the affinity tag is generally by linking the glycosyl acceptor to the affinity tag through its reducing end. Any suitable affinity tag can be used in this process. In some examples of the invention, affinity tags can be attached to insoluble polymers. For example, azides (Figure 4) or ketones / aldehydes can be used as affinity tags. After the synthesis of oligosaccharides is completed, the insoluble polymer is added to the reaction solution to connect the oligosaccharides to the polymer. Any insoluble polymer suitable for attachment to oligosaccharides can be used, such as phosphine-containing polymers. The polymer can be added in any suitable manner. For example, after oligosaccharide synthesis is complete, the polymer can be added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com