Preparation method of carbohydrate derivative by utilization of glycosyl transferase
A technology of glycosyltransferase and a manufacturing method, which is applied in the field of manufacturing carbohydrate derivatives, can solve the problems of high price of amylase, and achieve the effects of improved functional properties and huge application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
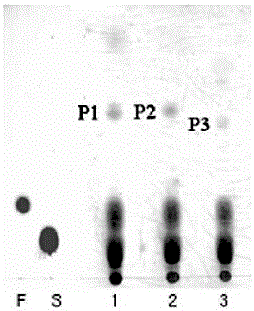
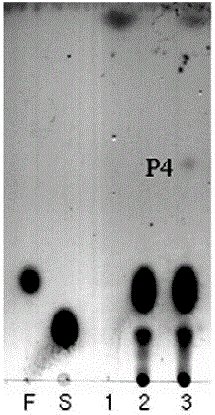
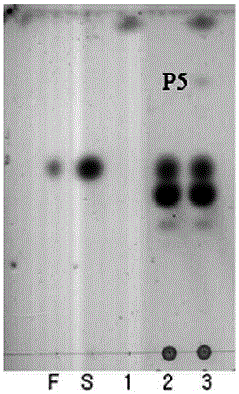
Image
Examples
Embodiment 1
[0045] The method for producing carbohydrate compound derivatives using glycosyltransferase of the present invention comprises the following steps: adding glycosyltransferase to a solution with a sugar concentration of 0.1M so that the final active concentration of the reaction vessel reaches 0.1U / mL Finally, add at least one glycosyl acceptor selected from the group consisting of 2-amino acid, 3-amino acid, 4-amino acid, hydroquinone and arbutin in the reaction kettle at 15°C to make the final activity The concentration reaches 0.01-3% by mass and reacts. The reaction is formed after 1 hour at 28 ° C. The glucose or fructosyl of the sugar is transferred to the above-mentioned acceptor, and finally acidified by acetic acid to obtain sugar compounds. derivative.
Embodiment 2
[0047] A method for producing carbohydrate compound derivatives utilizing glycosyltransferase, comprising the following steps: adding glycosyltransferase to a solution with a sugar concentration of 0.3M so that the final active concentration of the reactor reaches 0.5 U / mL, and then Add at least one glycosyl acceptor selected from the group consisting of 2-amino acid, 3-amino acid, 4-amino acid, hydroquinone and arbutin in a reaction kettle at 20°C, so that the final active concentration reaches The mass percentage content is 0.01-3% and reacted. The reaction is formed after 8 hours at 32 ° C. The glucose or fructosyl of the sugar is transferred to the above-mentioned acceptor, and finally the sugar compound derivative can be obtained by acetic acidification.
Embodiment 3
[0049] The method for producing carbohydrate compound derivatives using glycosyltransferase of the present invention comprises the following steps: adding glycosyltransferase to a solution with a sugar concentration of 0.5M so that the final active concentration of the reaction vessel reaches 2.0 U / mL Finally, add at least one glycosyl acceptor selected from the group consisting of 2-amino acid, 3-amino acid, 4-amino acid, hydroquinone and arbutin in the reaction kettle at 25°C, so that the final The active concentration reaches 0.01-3% by mass and reacts. The reaction is formed after 10 hours at 34°C. The glucose or fructosyl of the sugar is transferred to the above-mentioned acceptor, and finally the sugar can be obtained by acidification with acetic acid Compound derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com