Thermostable beta-galactosidase and application thereof in synthesis of glycerol galactoside
A technology of β-galactosidase and expression vector, which is applied in the application field of heat-stable β-galactosidase and its synthesis of glycerol galactoside, can solve the problems of complex product components, increased purification burden, and low reaction cost, and achieve Effect of high reaction temperature, good tolerance and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Construction of engineering bacteria for expressing galactosidase
[0023] The β-galactosidase source strain of the present invention is Bifidobacterium thermophilum NJ-5, and its amino acid sequence is shown in SEQ ID NO: 1. In order to improve the protein expression, a recombinant Escherichia coli expression vector is constructed.
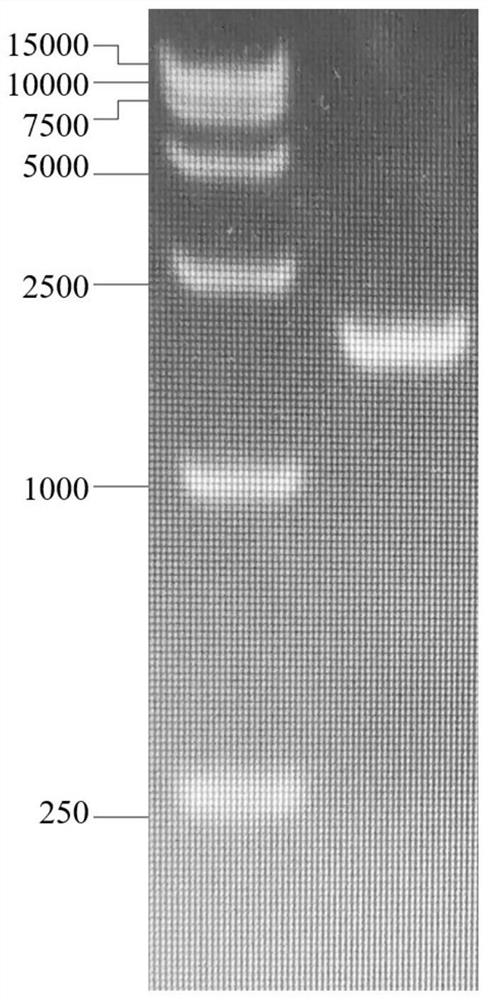
[0024] Using strain Bifidobacterium thermophilum NJ-5 genomic DNA as template, high-fidelity enzyme 2 × Phanta Max Master Mix (Nanjing Novizan Biotechnology Co., Ltd.), and primer pair B-F (5′CG GGATCC ATGACAGCACGCAGAACACATCG 3', BamH I, SEQ ID NO: 3) and B-R (5' CCG CTCGAG TCAACCCATGCTGACGATGACG 3'Xho I, SEQ ID NO: 4) was used for PCR amplification, and the experimental operation was referred to Vazyme biological products and operation manual. For nucleic acid electrophoresis of PCR products, see figure 1 , the DNA fragment of the coding gene obtained by amplification should be 2000bp, and the nucleic acid electrophoresis ve...
Embodiment 2
[0027] Example 2 Expression of β-galactosidase BtGal42 in Escherichia coli
[0028] The recombinant strain constructed in Example 1 was inoculated into 50 mL of LB liquid medium containing 100 μg / mL kanamycin sulfate, and cultured at 37° C. at 180 rpm overnight; the seed liquid was inoculated into fresh 50mL of LB liquid medium, 37°C, 180rpm to OD 600 When the temperature is 0.6 to 1.0, take it out and cool it in an ice-water bath for 5 min, add the inducer IPTG (isopropyl-β-D thiogalactoside) (final concentration 0.5 mmol / L), and induce expression for 20 h at 20 °C and 150 rpm.
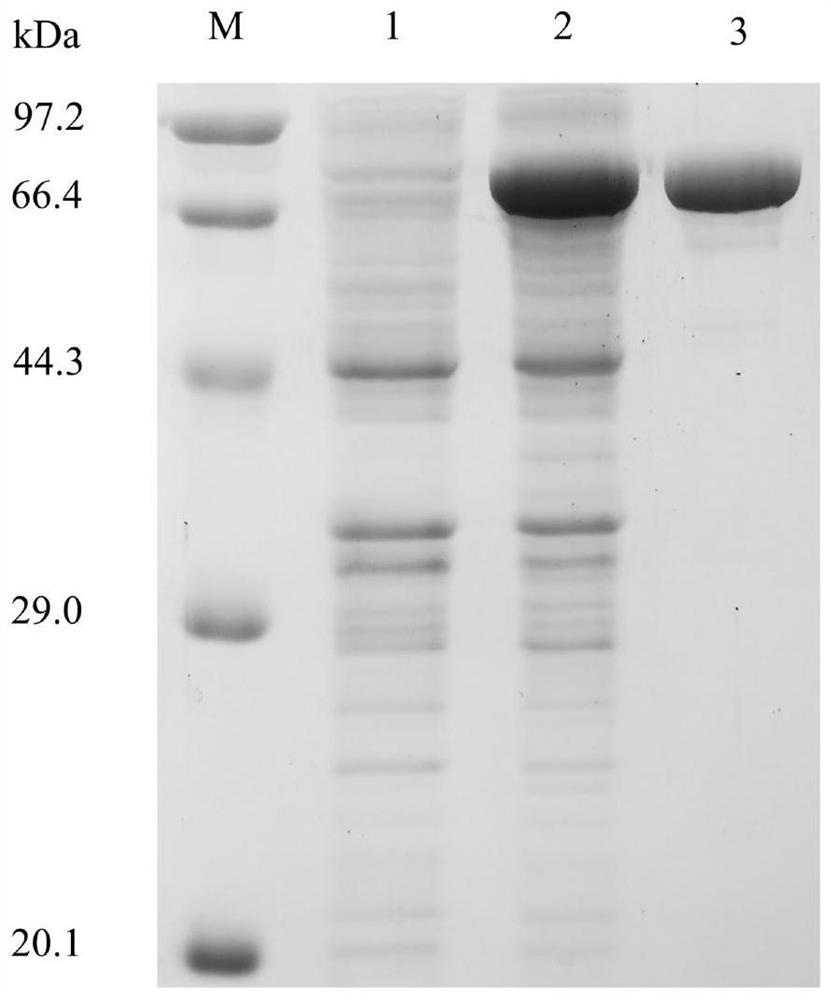
[0029] Take the induced expression fermentation broth, centrifuge at 12000rpm for 20min, discard the supernatant, and then use 50mM Na 2 HPO 4 -KH 2 PO 4(pH 7.0) buffer, resuspend and wash the cells, centrifuge at 12,000 rpm for 20 min, discard the supernatant, resuspend with buffer, and then sonicate. The broken liquid was centrifuged at 12000rpm for 20min, and the supernatant was taken for SDS...
Embodiment 3
[0031] Example 3 Determination procedure of galactosidase BtGal42 enzymatic activity
[0032] The recombinant strain constructed in Example 1 was fermented and cultivated according to the method of Example 2, and the obtained crude protein enzyme liquid was measured with β-oNPG as a substrate to measure the change of enzyme activity, and the measurement method was as follows:
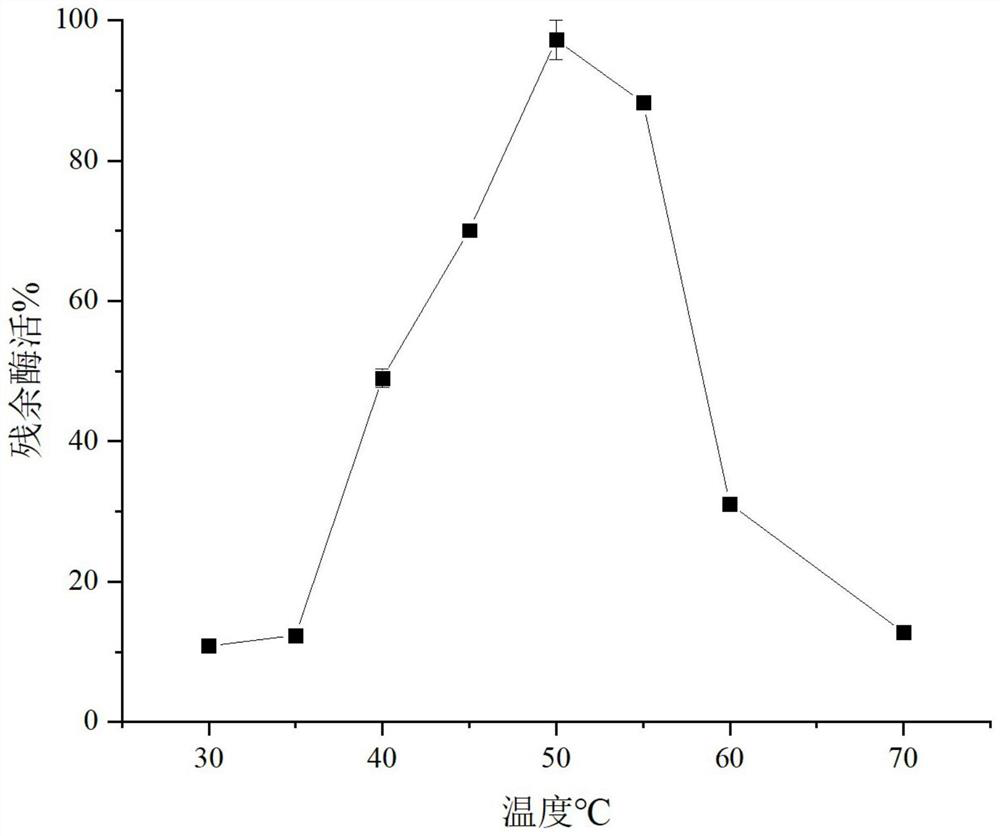
[0033] Definition of enzyme activity unit: One enzyme activity unit is the amount of enzyme required to catalyze the hydrolysis of β-oNPG to generate 1 μmol oNP per minute at 50°C and pH 7.0.
[0034] Accurately weigh 30 mg of β-oNPG and dissolve it in 10 mL of Na 2 HPO 4 -KH 2 PO 4 Buffer (50mM, pH 7.0), stir and mix to obtain a substrate solution with a concentration of 10mmol / L. Accurately pipette 240 μl of the substrate solution into a 96-well plate, add 10 μl of appropriately diluted enzyme solution, take the inactivated enzyme reaction solution as a control, and react at 50 °C for 10 min, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com