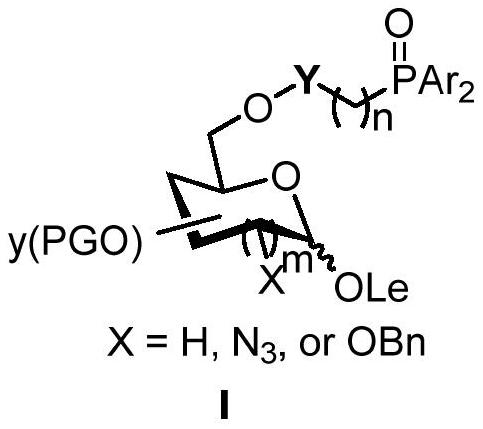
Stereoselective synthesis method of beta-2-deoxysugar, 2-deoxy-2-azide sugar and glucoside bond
A glucosidic bond and stereoselectivity technology, applied in chemical instruments and methods, organic chemical methods, sugar derivatives, etc. Wide range, high flexibility and universality, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Compound III-1
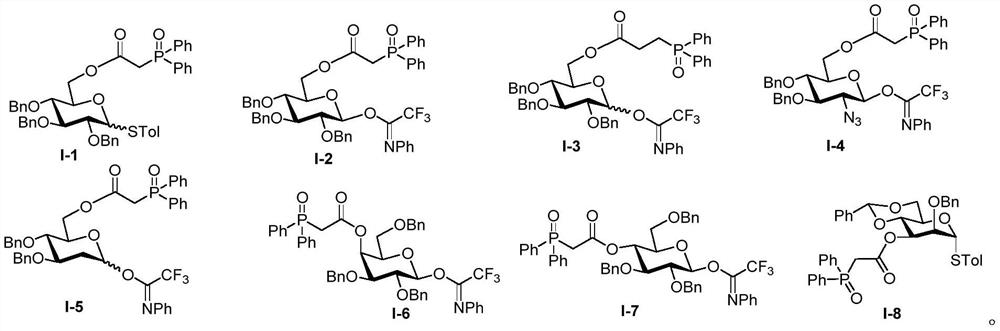
[0062] The donor Ⅰ-2 (50mg, 0.06mmol) and the corresponding acceptor (14mg, 0.05mmol) were dissolved in 2mL of dry toluene, and TMSOTf (1.4μL, 0.006mmol) was used as a catalyst to obtain compound Ⅲ-1 (41mg, 93%, β / α>20:1), white solid: 1 H NMR (500MHz, CDCl 3 )δ7.81–7.74(m,5H),7.51–7.39(m,10H),7.34–7.26(m,13H),7.18(d,J=7.8Hz,2H),5.56(d,J=5.0Hz ,1H),5.50(d,J=5.0Hz,1H),5.03(d,J=11.2Hz,1H),4.95(d,J=11.0Hz,1H),4.77–4.71(m,3H),4.59 (dd, J=7.9,2.3Hz,1H),4.42(d,J=10.9Hz,1H),4.37(d,J=7.8Hz,1H),4.31(dd,J=4.9,2.3Hz,1H) ,4.25–4.21(m,2H),4.14(dd,J=11.8,3.6Hz,1H),4.06(dd,J=11.4,3.6Hz,2H),3.68(dd,J=11.6,8.5Hz,1H ),3.59–3.54(m, 1H),3.49(dd,J=12.7,10.7Hz,2H),3.33(dd,J=20.7,11.9Hz,3H),1.72(s,3H),1.50(s, 3H), 1.44(s,3H), 1.31(s,3H), 1.31(s,3H); 13 C NMR (125MHz, CDCl 3 )δ165.8,165.8,138.7,138.6, 137.9,132.3,132.3,131.5,131.4,131.2,131.2,131.1,131.1,128.8,128.7,128.7,128.6,128.4, 128.3,128.2,128.1,127.8,127.8,127.5,109.4 ,108.6,104.3,96.4,84.4,81.2,77.2,...
Embodiment 2
[0063] Example 2 Compound III-2
[0064] The donor Ⅰ-4 (30mg, 0.038mmol) and the corresponding acceptor (8.2mg, 0.031mmol) were dissolved in 2mL of dry toluene, and TMSOTf (0.68μL, 0.0031mmol) was used as a catalyst to obtain compound Ⅲ-2 (26mg ,94%,β / α>20:1), white solid: 1 H NMR (500MHz, CDCl 3 )δ7.83(dd,J=11.6,7.5Hz,4H),7.56–7.49(m,6H), 7.41–7.33(m,8H),7.27–7.22(m,2H),5.59(d,J= 5.0Hz, 1H), 4.94(d, J=10.9Hz, 1H), 4.78(dd, J=16.4, 10.9Hz, 2H), 4.65(dd, J=7.9, 2.2Hz, 1H), 4.50(d, J=10.9Hz, 1H), 4.40(d, J=7.9Hz, 1H), 4.36(dd, J=5.0, 2.3Hz, 1H), 4.30(dd, J=14.4, 6.6Hz, 2H), 4.20( dd,J=11.9,4.0Hz,1H), 4.11–4.02(m,2H),3.78(dd,J=11.1,6.8Hz,1H),3.57–3.50(m,2H),3.42–3.33(m, 4H), 1.59(s, 3H), 1.47(s, 3H), 1.39(s, 3H), 1.37(s, 3H); 13 C NMR (125MHz, CDCl 3 )δ166.0,166.0,138.3, 138.0,132.6,132.5,132.4,131.6,131.4,131.4,131.3,131.3,129.1,129.0,128.9,128.7,128.7, 128.3,128.2,128.2,128.1,109.6,108.9,102.5,96.6 ,83.2,77.2,75.2,73.0,71.5,71.0,70.8,69.1, 67.9,66.5,64.0,39.4,38.9,26.3,2...
Embodiment 3
[0065] Example 3 Compound III-3
[0066] The donor Ⅰ-5 (30mg, 0.042mmol) and the corresponding acceptor (9.2mg, 0.035mmol) were dissolved in 2mL of dry toluene, and TMSOTf (0.68μL, 0.0031mmol) was used as a catalyst to obtain compound Ⅲ-3 (27mg ,92%, β / α=10:1), as a white solid: 1 H NMR (300MHz, CDCl 3 )δ7.79(dd, J=12.0,5.7Hz,4H),7.48(dd,J=7.5,2.9Hz,6H),7.31(dd,J=10.5,5.1Hz,8H),7.25–7.22(m ,2H),5.55(d,J=5.0Hz,1H),5.50(d,J=5.2 Hz,1H),4.83–4.77(m,1H),4.68–4.62(m,1H),4.59(dd, J=8.0,2.4Hz,1H),4.54(d,J=11.6Hz,1H),4.45(dd,J=9.5,6.0Hz,2H),4.31(dd,J=5.0,2.3Hz,1H), 4.24–4.14(m,3H),3.99(dd,J=11.9,3.3Hz,2H),3.61(dd,J=11.8,8.4Hz,2H),3.53(d,J=4.6Hz,1H),3.48 (d,J=4.4Hz,1H), 3.31–3.27(m,2H),2.43(dd,J=11.7,4.7Hz,1H),1.59(d,J=11.9Hz,1H),1.54(s, 3H), 1.43(s, 3H), 1.34(s, 3H), 1.31(s, 3H); 13 C NMR (125MHz, CDCl 3 ) Δ165.9,138.2,138.2,132.2,131.2, 131.2,131.1,131.1,128.7,128.7, 128.6,128.4,127.8, 127.7.7.4, 100.4,96.7,7.2,7.2,7.2,7.2,7.2,7.2,7.2,7.2,7.7.2,7.2,7.2,7.2,7.7.2,7.7. , 71.4, 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com