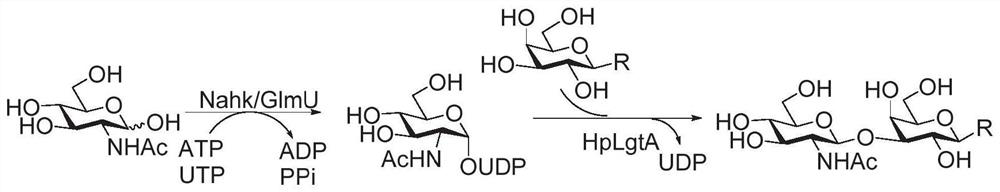
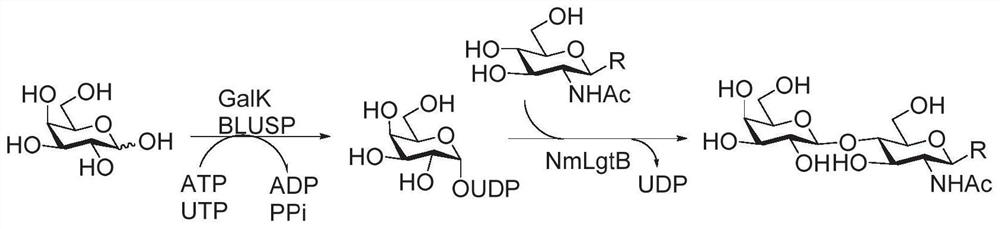
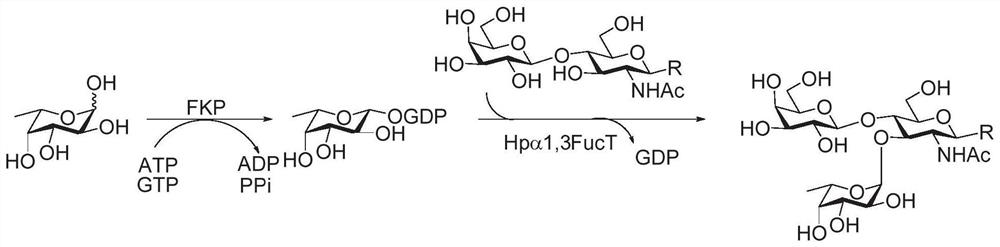
Fluorine label and preparation method thereof, and method for synthesizing oligosaccharide chain by adopting assisted enzymic method
An enzymatic synthesis, oligosaccharide chain technology, applied in the preparation of sugar derivatives, chemical instruments and methods, oligosaccharides, etc., can solve problems such as difficulty in large-scale use, limited reaction scale, compound loss, etc. Wide adaptability, high assembly efficiency, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: A method for rapid separation of oligosaccharides assisted by fluorine tags
[0085] Proceed as follows:
[0086] (1) Chemical synthesis of lactoside 1 (formula IV) in the β-configuration;
[0087] Add lactose 11 (10 g, 29.23 mmol), acetic anhydride (55 mL) and sodium acetate (9.6 g) into a 500 mL round bottom flask, and stir under reflux at 160° C. for 6 hours. Thin-layer chromatography (PE:EA=1:2) detected that the reaction was complete, and concentrated by rotary evaporation. The resulting solid was redissolved in 250 mL of dichloromethane, extracted twice with half-saturated saline, three times with saturated sodium bicarbonate solution, and three times with double-distilled aqueous solution, then separated the organic phase, dried the organic phase with anhydrous sodium sulfate, and spun Concentrated by evaporation to obtain light yellow solid compound 12 (18.70 g, 94%).
[0088] Add compound 12 (15.0g, 22.12mmol) ammonium acetate (6.8g, 88.21mmol) me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com