Enzymatic modules and Sda carbohydrate antigen synthesis method
A synthetic method and technology for carbohydrate antigens, applied in the field of carbohydrate antigen preparation, can solve the problems of low overall yield, low yield, and narrow substrate applicability, and achieve the effects of strong catalytic specificity, high catalytic efficiency, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
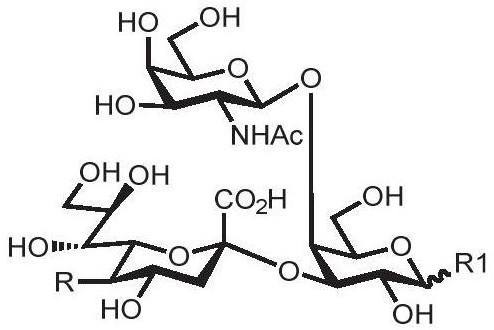
[0089] 1. Chemical synthesis of α-configuration disaccharide compound 3 (GlcNAcβ1,4GalNAcαproN 3 )
[0090] In a 50mL double-necked round bottom flask, the glycosyl acceptor (compound 1) (455mg, 1.16mmol), the glycosyl donor (compound a) (1.08g, 1.74mmol) and dry Molecular sieves were mixed in dry dichloromethane, sealed, filled with argon protection and stirred at room temperature for 30 minutes. Subsequently, the reaction system was cooled to 0°C in a low-temperature reactor, and the accelerator trifluoromethanesulfonic acid (TfOH, 30 μL, 0.35 mmol) was added. After two hours, thin-layer chromatography detected (petroleum ether: ethyl acetate=1:3 , v / v) The receptors basically responded completely. The reaction system was diluted with dichloromethane and filtered with diatomaceous earth. The obtained organic phase was extracted with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, concentrated by rotary evaporation, and then separated and purifi...
Embodiment 2
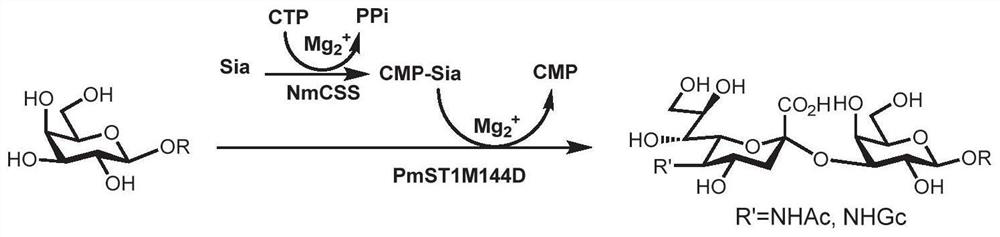
[0104] Enzymatic module A method for synthesizing sialylated products 11, 12, 14, 15:
[0105] Add acceptor compound (compound 10 or compound 13) (1.0 equiv), sialic acid (Neu5Ac or Neu5Gc, 1.1 equiv), cytidine triphosphate (CTP, 1.2 equiv), Tris-HCl buffer ( 100mM, pH=8.0) and magnesium chloride (20mM), add double distilled water to adjust the volume to 10mL, shake and mix, add enzyme NmCSS, PmST1M144D. The reaction was carried out at 37° C. and 110 rpm for 12 hours. Thin-layer chromatography detection (ethyl acetate: methanol: water: acetic acid = 4:2:1:0.2, v / v) After the reaction was completed, an equal volume of ice ethanol 10 mL was added to terminate the reaction, and the reaction was left to stand at 4°C for 30 minutes. The reaction system was centrifuged at 4° C. and 10,000 rpm for 10 minutes, the supernatant was concentrated by spinning to dryness, and separated and purified by Bio-Gel P2 gel molecular exclusion chromatography to obtain the product.
[0106] The sy...
Embodiment 3
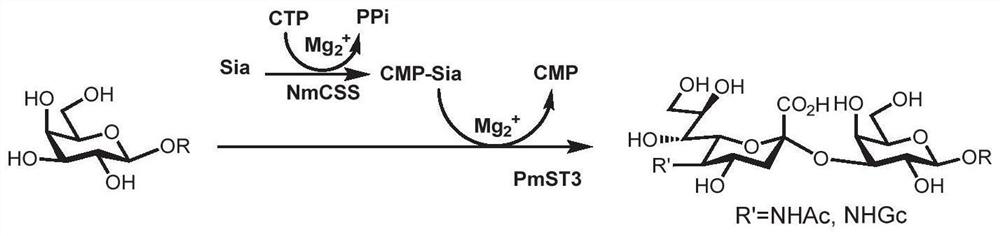
[0117] Enzymatic Module B method for sialylation of products 17–22, 24, 25:
[0118] Add acceptor compound (compound 4, compound 9, compound 16 or compound 22) (1.0 equivalent), sialic acid (Neu5Ac or Neu5Gc, 1.1 equivalent), cytidine triphosphate (CTP, 1.2 equivalent) to a 50 mL centrifuge tube, Tris-HCl buffer (100mM, pH=8.0) and magnesium chloride (20mM), add double distilled water to adjust the volume to 10mL, shake and mix, add enzymes NmCSS and PmST3. The reaction was carried out at 37° C. and 110 rpm for 12 hours. TLC detection (ethyl acetate: methanol: water: acetic acid = 4:2:1:0.2, v / v). After the reaction was completed, an equal volume of 10 mL of ice ethanol was added to terminate the reaction, and the mixture was left to stand at 4° C. for 30 minutes. The reaction system was centrifuged at 4° C. and 10,000 rpm for 10 minutes, the supernatant was concentrated by spinning to dryness, and separated and purified by Bio-Gel P2 gel molecular exclusion chromatography t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com