Microbes for alkyl glycoside synthesis and application thereof
A technology of alkyl glycosides and microorganisms, which is applied in the field of alkyl glycoside synthesis and can solve the problems of undiscovered glucosidase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
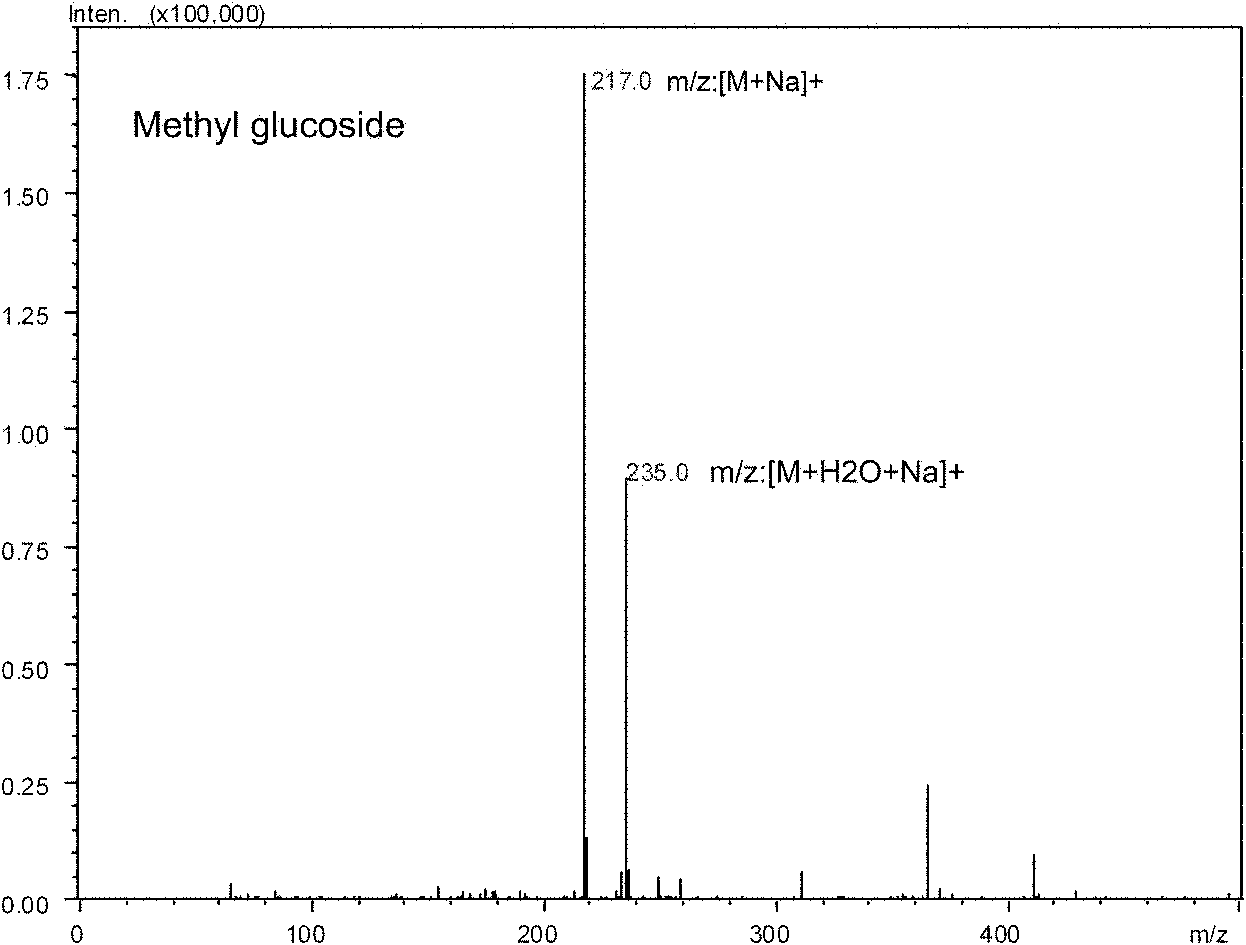
[0038] Synthesis of methylglucoside catalyzed by Arthrobacter sp.DL001
[0039] The bacteria were inserted into the liquid medium at 120 rpm, cultured at 10°C for 60 hours, and then stored at -20°C.
[0040] The liquid medium used is: dextran 8g, KNO 3 1.2g, K 2 HPO 4 · 3H2O 1.5g, water 1000mL, NaOH to adjust the pH to 6.5-7.0.
[0041] Take 180 μl of the above fermentation broth, add 20 μl of 2% (w / v, the same below) starch solution and 10 μl of methanol, react in a constant temperature water bath at 20°C for 24 hours, then add an equal volume of 200 μl of methanol to terminate the reaction, centrifuge at 15000g*5min*4°C, and place on Qing is the product of transglycosylation.
[0042] UFLC-MS identification was carried out on the supernatant obtained above, and the yield of the obtained product methyl glucoside was about 30%. The equipment and conditions used for the above UFLC-MS analysis: ultra-fast liquid chromatography (UFLC) system including CBM-20A system controll...
Embodiment 2
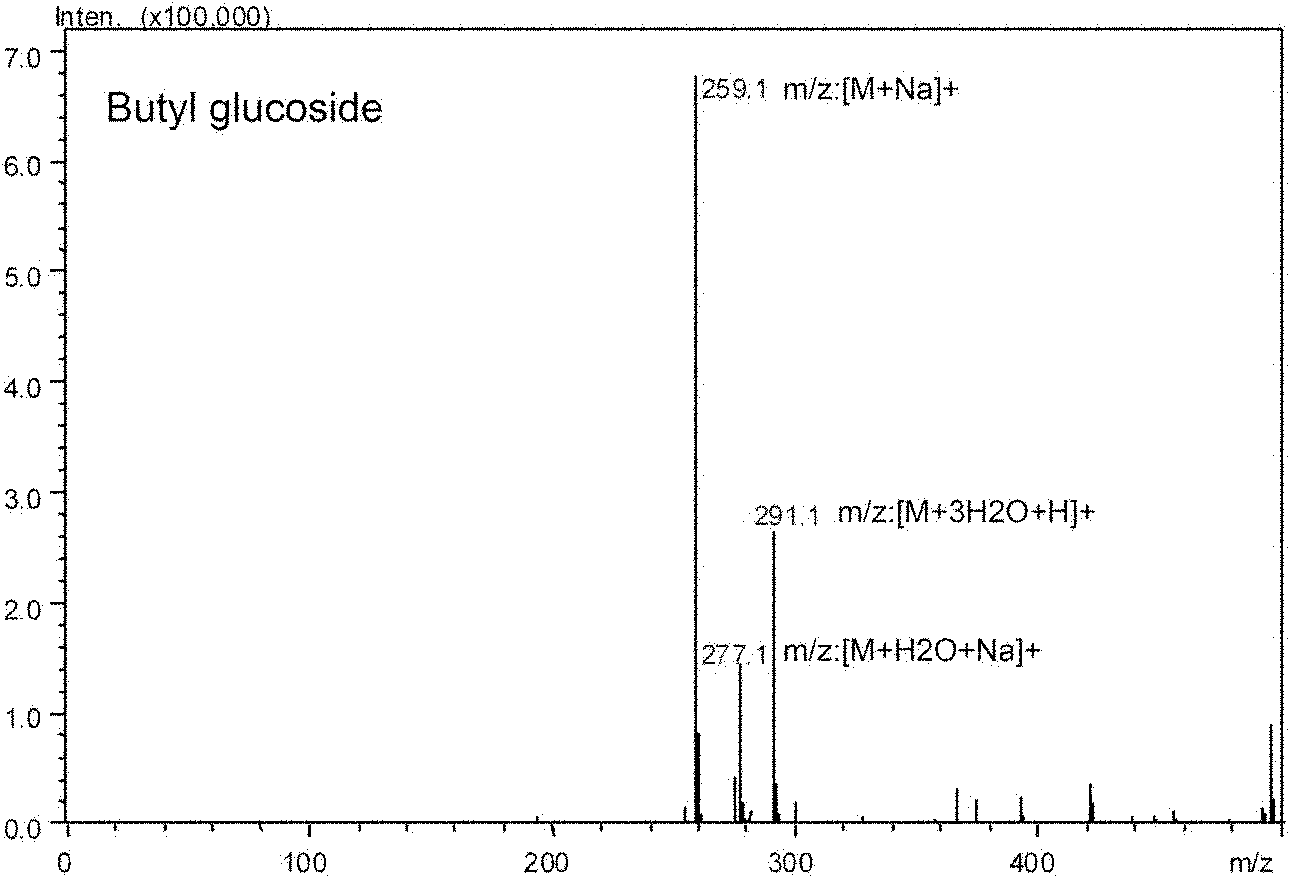
[0044] The synthesis reaction of n-butyl glucoside catalyzed by Arthrobacter sp.DL001 put the bacteria into the liquid medium at 200rpm, culture at 30°C for 45h, centrifuge the fermentation broth, and take the supernatant, which is the crude enzyme of glycosyltransferase solution, stored at -20°C.
[0045] The liquid medium used is maltose 20g, KNO 3 1g, K 2 HPO 4 ·3H 2 O 0.5g, water 1000mL, NaOH to adjust the pH to 7.4-7.6.
[0046] Take 1.8ml of the above fermentation broth, add 0.1ml of 10% (w / v, the same below) maltose, 0.1ml of n-butanol, react in a constant temperature water bath at 25°C for 5h, then add an equal volume of 2ml of methanol to terminate the reaction, centrifuge at 15000g*5min*4°C Finally, the supernatant is the transglycosylated product.
[0047] UFLC-MS identification was performed on the above-mentioned supernatant, and the equipment and parameters were the same as before. The yield of the obtained product n-butyl glucoside is about 50%.
Embodiment 3
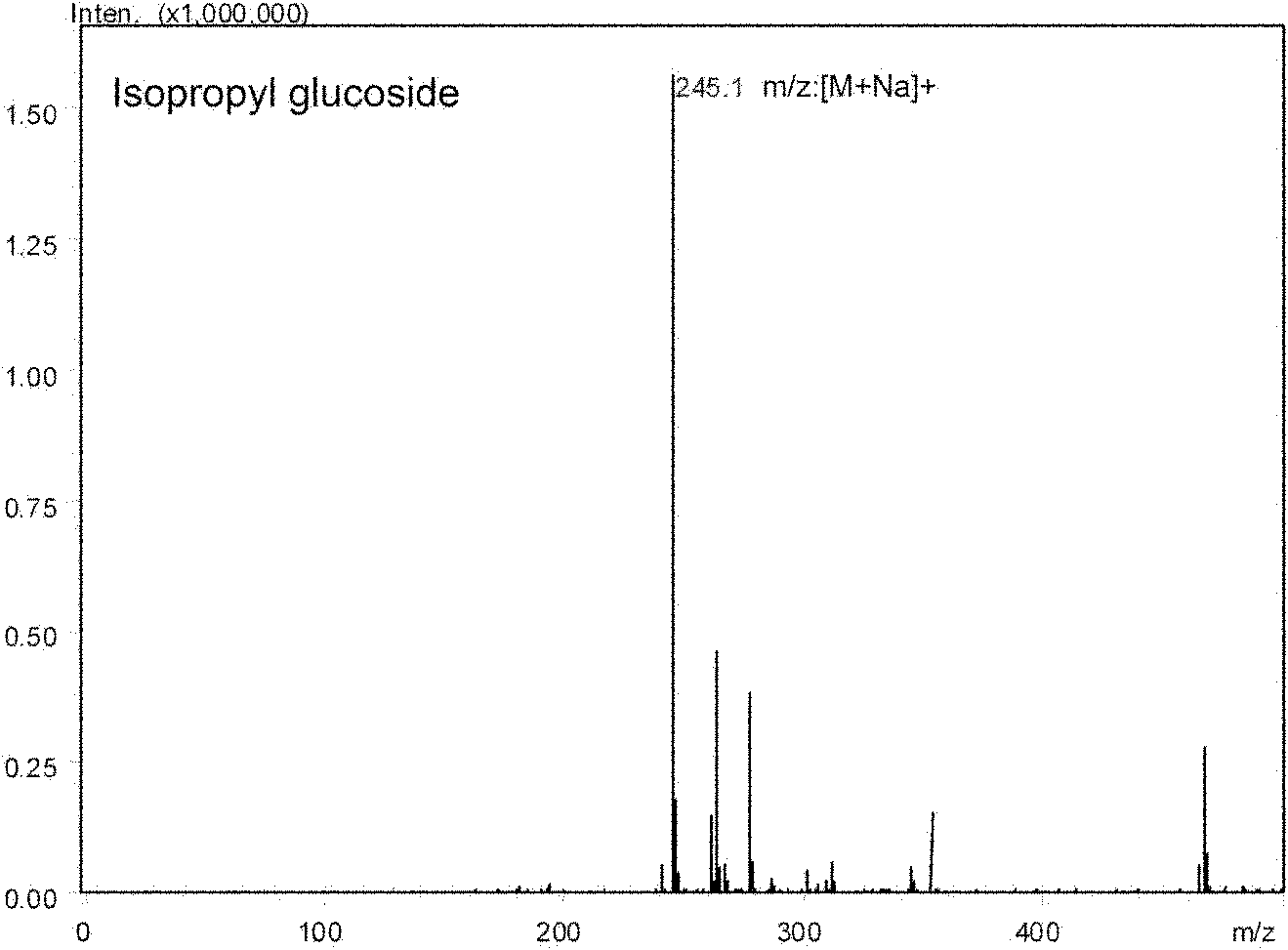
[0049] Synthesis of Isopropylglucoside Catalyzed by Arthrobacter sp.DL001
[0050] Put the bacteria into the liquid medium at 250 rpm, culture at 35°C for 48 hours, centrifuge the fermentation broth, take the supernatant, which is the crude glycosyltransferase enzyme solution, and store it at -20°C.
[0051] The liquid medium used is: glucose 40g, NaNO 3 0.8g, K 2 HPO 4 ·3H 2 O 2g, water 1000mL, NaOH to adjust the pH to 7.0-7.5.
[0052] Take 180 μl of the above fermentation broth, add 10 μl of 20% (w / v, the same below) maltose, 5ul of isopropanol, react in a constant temperature water bath at 30°C for 2 hours, then add an equal volume of 200ul of methanol to terminate the reaction, centrifuge at 15000g*5min*4°C, The supernatant is the transglycosylated product.
[0053] UFLC-MS identification was performed on the above-mentioned supernatant, and the equipment and parameters were the same as before. The yield of the product isopropylglucoside was about 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com