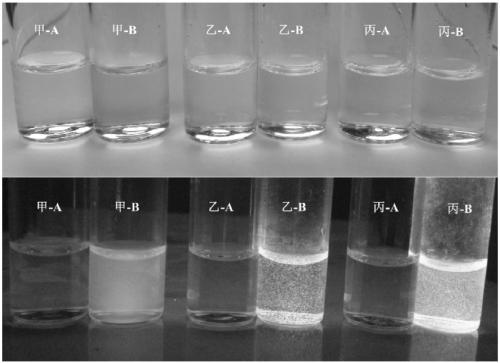
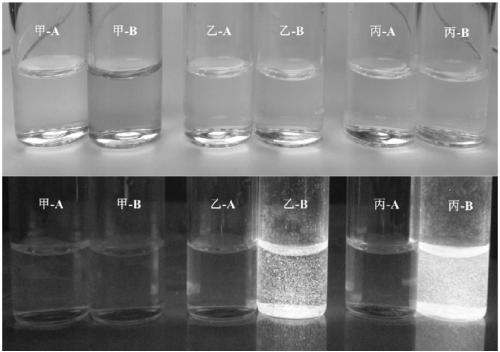
A kind of synthetic method of the glycoside based on indoxyl derivative, 2-(benzothiazol-2'-yl)phenol derivative
A synthetic method, benzothiazole technology, applied in the field of glycoside synthesis, can solve problems such as poor solubility, many colored impurities, complex reaction mixture, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of (5-bromo-4-chloroindol-3-yl) β-D-glucoside (X-Glu)
[0034] Horwitz et al. have reported that (5-bromo-4-chloroindol-3-yl) acetate derivatives were used as intermediates in the preparation of the glycosides (see formula 1b) Under the condition of strict anaerobic, anhydrous and 0°C, the 3-position acetyl group was first removed, and then the methanol solution containing acetyl bromide-α-D-glucose was added to react for 18 hours. During this period, anaerobic, anhydrous and At 0°C, this method completes the glycosidation and deprotection reactions in "one pot", with a yield of 25% (J.Med.Chem., 1964, 7, 574-575). etc. have reported using 3-hydroxyindole-2-carboxylate derivatives (see formula 1c) as intermediates to synthesize the glycoside (Eur.J.Org.Chem., 2014, 564-574.).
[0035]
[0036] And the preparation method of the present invention is as follows:
[0037] (1) Glycosidation reaction
[0038] Under the protection of argon, containi...
Embodiment 2
[0048] Example 2: Synthesis of (5-bromo-4-chloroindol-3-yl) β-D-galactoside (X-Gal)
[0049] Horwitz et al. reported the synthesis of this glycoside, but did not describe the method used and its yield (J. Med. Chem., 1964, 7, 574-575). etc. have reported using 3-hydroxyindole-2-carboxylate derivatives (see formula 1c) as intermediates to synthesize the glycoside (Eur.J.Org.Chem., 2014, 564-574.). And the preparation method of the present invention is as follows:
[0050] (1) Glycosidation reaction
[0051] Under the protection of argon, containing 1-acetyl-5-bromo-4-chloroindolin-3-one (289mg, 1.00mmol), TBAHS (68mg, 0.20mmol) and K 3 PO 4 (1062mg, 5.00mmol) in the round bottom flask, add H successively 2 O [53.1 μL, 5% (w / w)] and CH 2 Cl 2 (10mL), stirred at room temperature for about 10min, then added acetyl bromide-α-D-galactose (1028mg, 2.50mmol) all at once, and the reaction was completed after stirring for 4h. The liquid was separated, and the obtained organic ph...
Embodiment 3
[0059] Example 3: Synthesis of (5-bromo-6-chloroindol-3-yl) β-D-galactoside (Magenta-Gal)
[0060] Horwitz et al. have reported that when preparing the glycoside, use 1-acetyl-5-bromo-6-chloroindolin-3-one and acetyl bromide-α-D-galactose in NaOH aqueous solution / acetone / 0℃ / N 2 Under the conditions of protection, the reaction was carried out for 16 hours, and the yield of glycosidation reaction was 49%; during the deprotection reaction, sodium methoxide / methanol / 5°C was used for alcoholysis overnight, and the yield was 86% (J.Med.Chem., 1964,7,574- 575). etc. have reported using 3-hydroxyindole-2-carboxylate derivatives (see formula 1c) as intermediates to synthesize the glycoside (Eur.J.Org.Chem., 2014, 564-574.).
[0061] And the preparation method of the present invention is as follows:
[0062] (1) Glycosidation reaction
[0063] Under the protection of argon, containing 1-acetyl-5-bromo-6-chloroindolin-3-one (289mg, 1.00mmol), TBAHS (68mg, 0.20mmol) and K 3 PO 4 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com