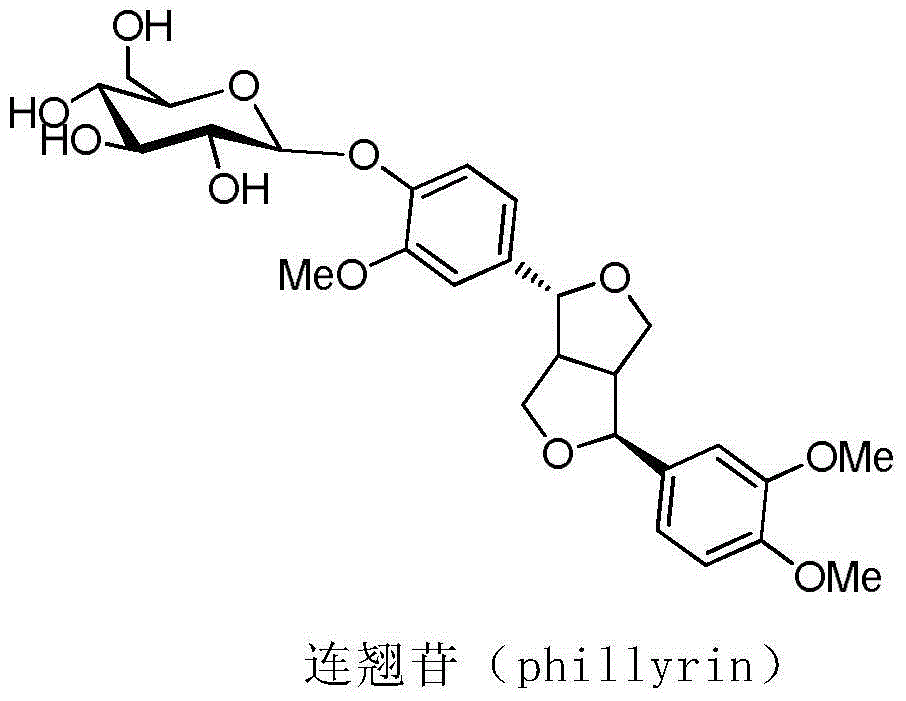
Chemical synthesis method of phillyrin
A technology for chemical synthesis and forsythin, applied in chemical instruments and methods, organic chemistry, pharmaceutical formulations, etc., can solve the problems of hydrobromic acid corrosiveness, unfavorable operation, low synthesis yield, etc., and achieve convenient source of raw materials and easy preparation The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
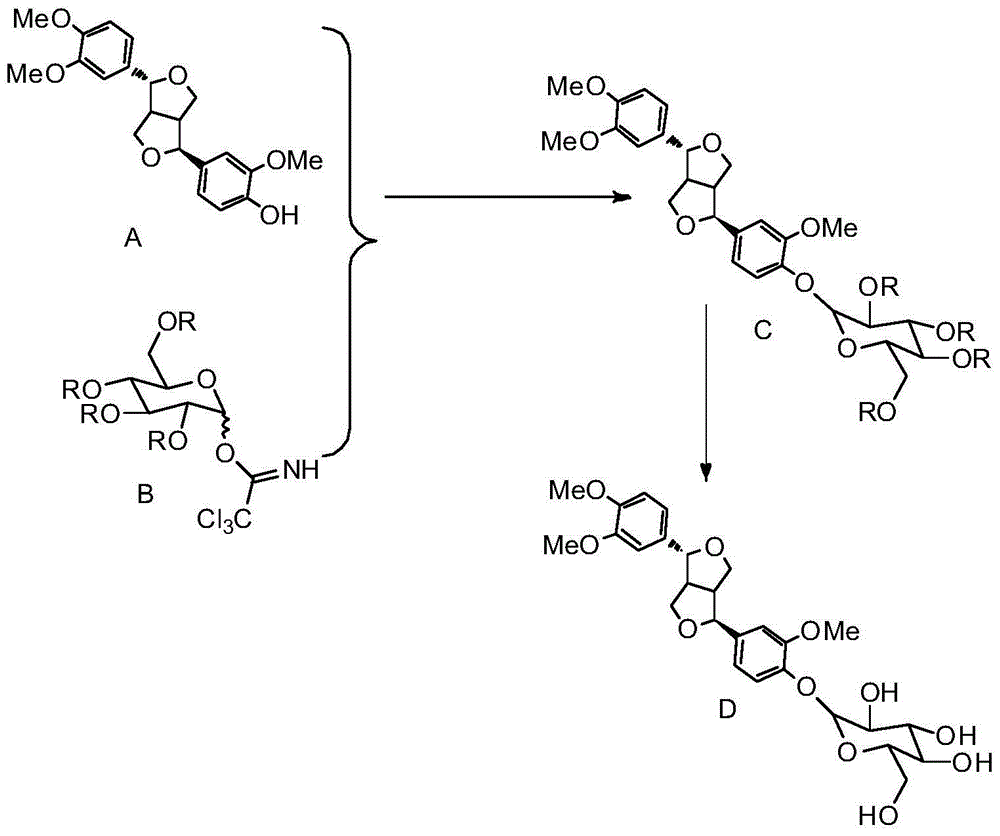
[0042] 1) Glycosidation reaction
[0043] Put forsythiatin (372mg, 0.001mol), 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate (738mg, 0.0015mol) in a 100mL three-necked flask , wherein the molar ratio of forsythiatin to 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate was 1:1.5, adding 20 mL of anhydrous dichloromethane , type aluminosilicate molecular sieve (744mg); then pass into inert gas nitrogen to carry out inert gas protection, stir for 0.5h, after mixing uniformly, add Lewis acid catalyst trimethylsilyl trifluoromethanesulfonate (TMSOTf, 0.06mL, 0.312 mmol), the molar ratio of Lewis acid catalyst to 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimide ester is 1:5, the mass of molecular sieve and forsythiatin The ratio is 2:1, stirring at 0°C for glycosylation reaction for 10 hours;
[0044] After the Lewis acid deprives the hydrogen of the hydroxyl group of the reaction substrate forsythiatin, the generated active intermediate will b...
Embodiment 2
[0058] 1) Glycosidation reaction
[0059] Put forsythiatin (372mg, 0.001mol), 2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate (1.11g, 0.0015mol) in 100mL In a three-necked flask, where the molar ratio of forsythiatin and 2,3,4,6-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate is 1:1.5, add 20 mL of anhydrous dichloromethane, Type aluminosilicate molecular sieve (744mg), then pass into inert gas argon to carry out inert gas protection, after stirring for 0.5h, dropwise add Lewis acid catalyst silver trifluoromethanesulfonate 80mg (0.312mmol), Lewis acid catalyst and 2,3 , The molar ratio of 4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate is 1:5, the mass ratio of molecular sieve to forsythiatin is 2:1, at 10℃ Stirring, carry out glycosylation reaction 8 hours;
[0060] Molecular sieves are added to remove the water generated by the reaction to ensure that the reaction proceeds in the positive direction.
[0061] 2) Quenching treatment
[0062]...
Embodiment 3
[0068] 1) Glycosidation reaction
[0069] Put forsythiatin (372mg, 0.001mol), 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate (1.23g, 0.0025mol) in 100mL three ports In the flask, the molar ratio of forsythiatin and 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate is 1:2.5, add 20 mL of anhydrous trichloro methane, Type aluminosilicate molecular sieve (744mg), then pass into inert gas nitrogen for inert gas protection, after stirring for 0.5h, add Lewis acid catalyst trimethylsilyl trifluoromethanesulfonate (0.08mL, 0.416mmol) dropwise, Lewis acid The molar ratio of catalyst to 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl trichloroacetimidate is 1:6, and the mass ratio of molecular sieve to forsythiatin is 2:1 , stirred at 0°C, and carried out glycosylation reaction for 10 hours;
[0070] 2) Quenching treatment
[0071] Add quencher triethylamine (0.416mmol) to the reaction mixture to quench the glycosylation reaction, the molar ratio of quencher triet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com