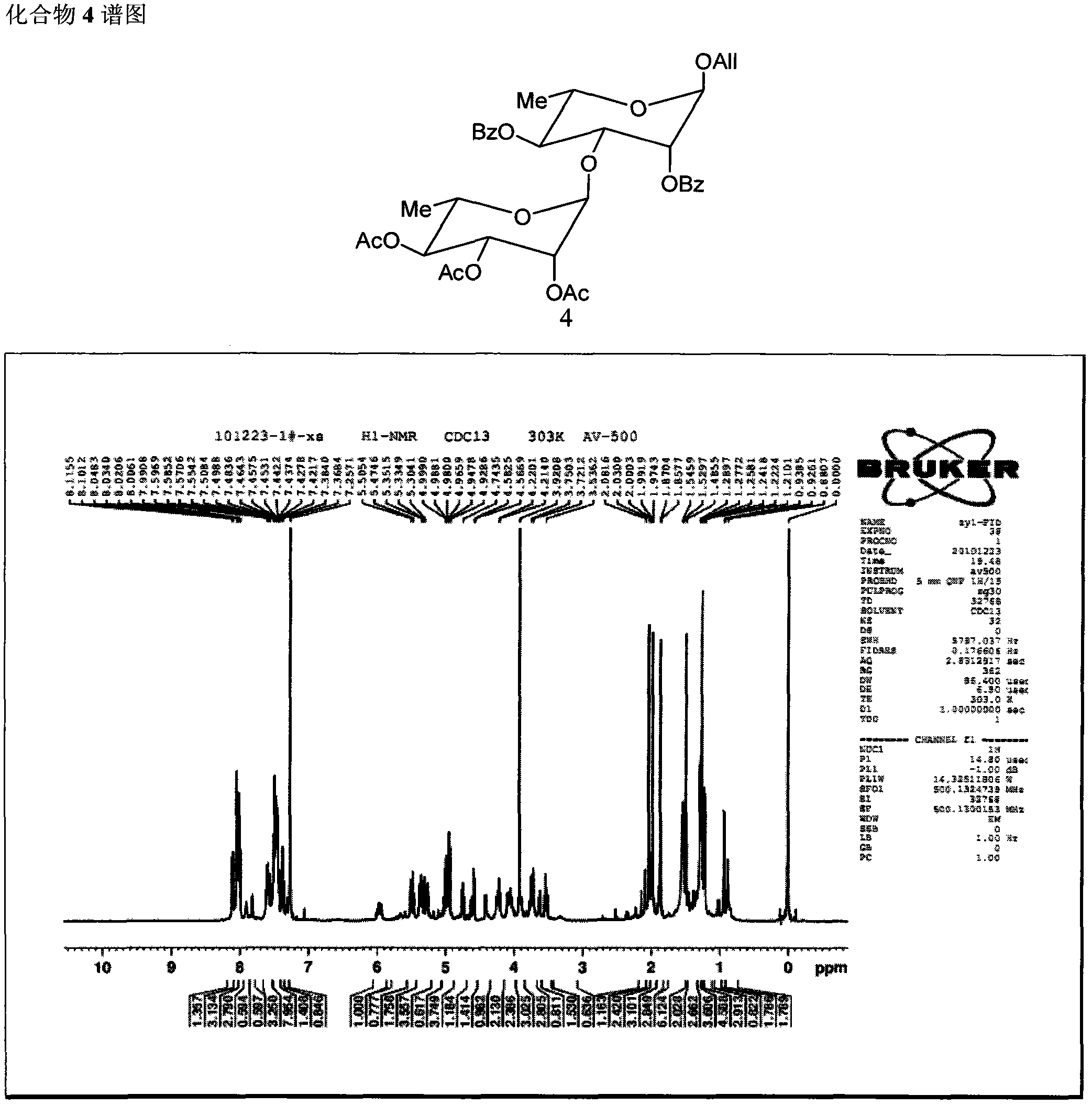
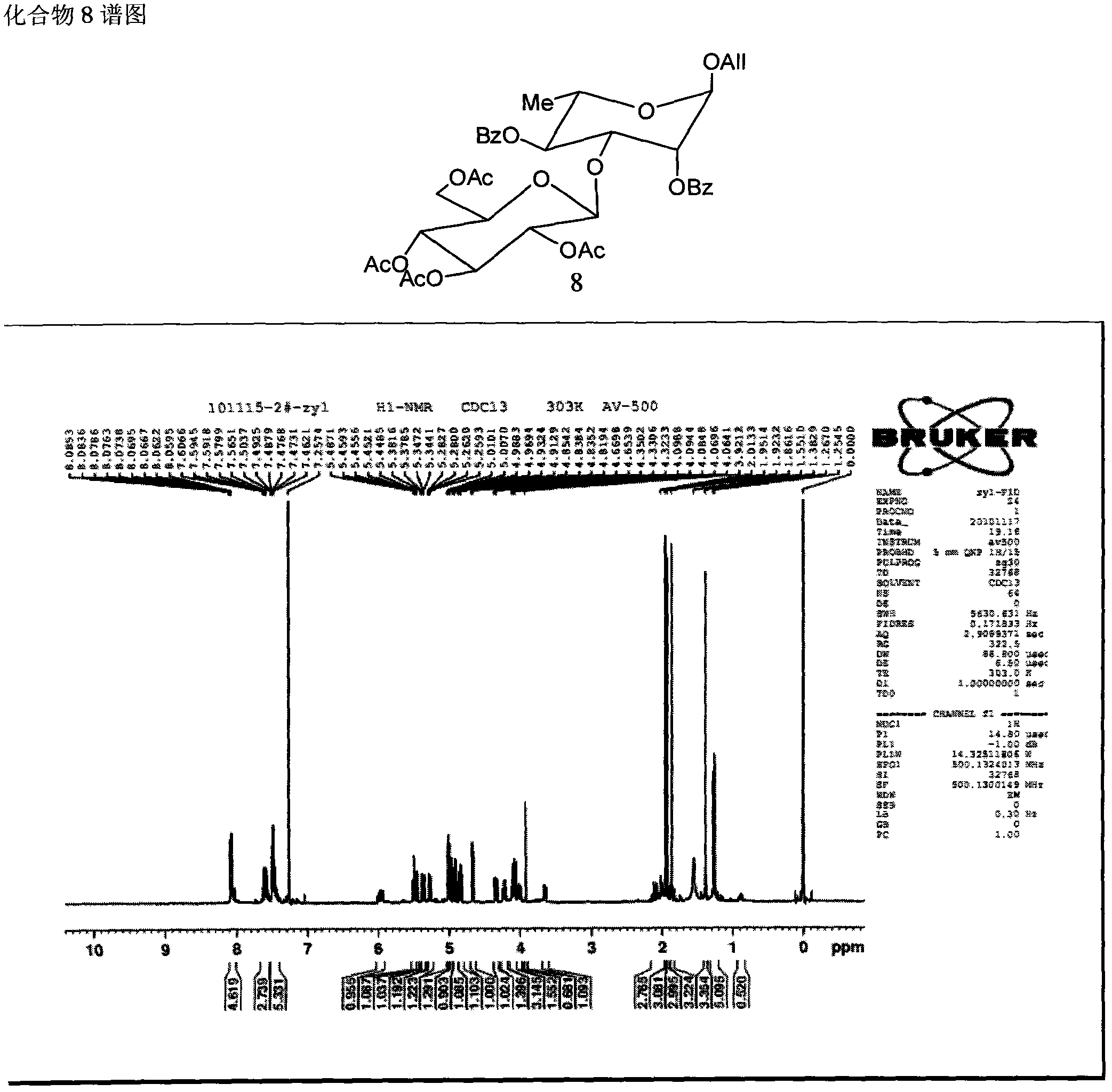
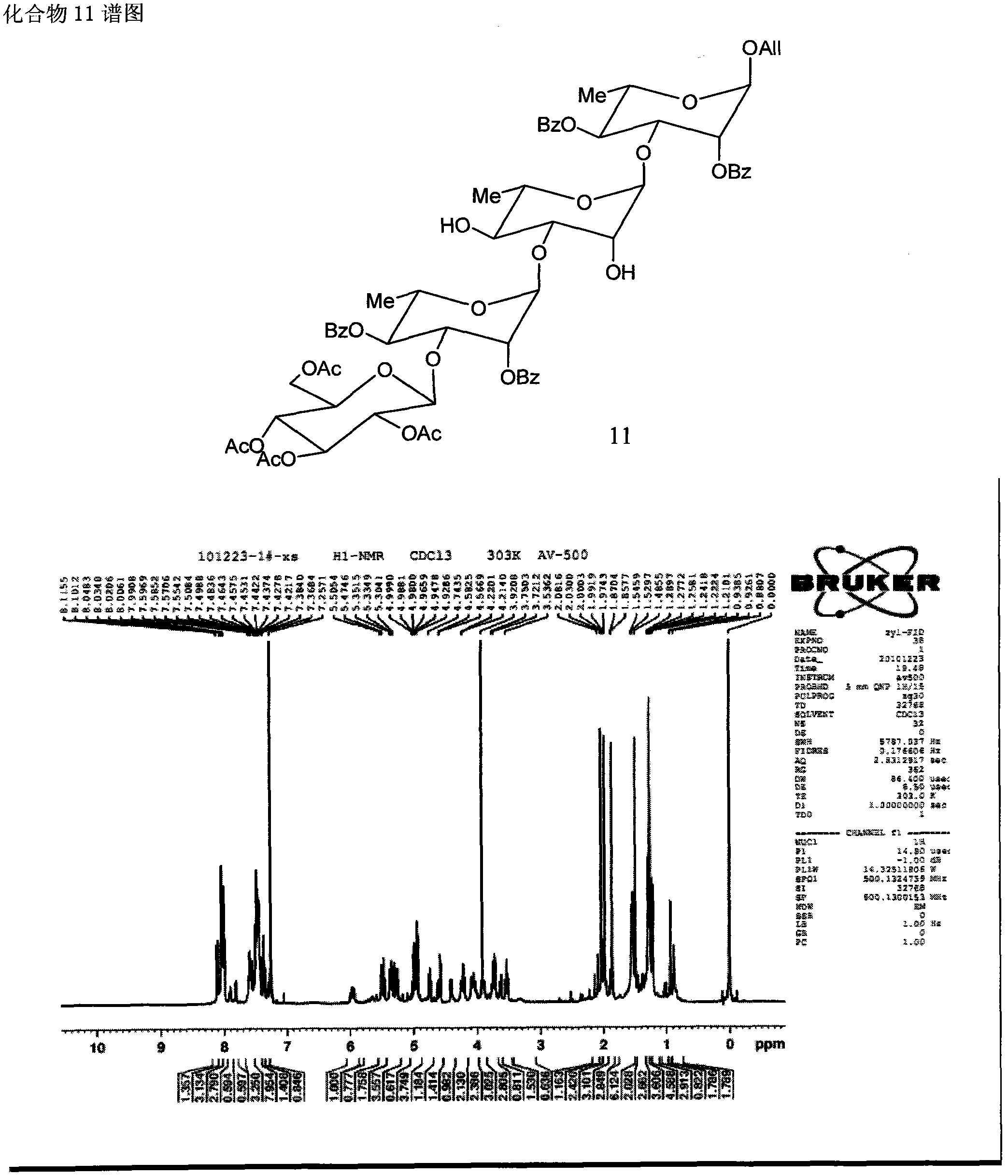
Method for synthesizing beta-D glucose(1->3)alpha-L rhamnose(1-3)alpha-L rhamnose(1-3)alpha-L rhamnose
A technology of rhamnose and glucose, which is applied in the field of synthesis of β-D glucose α-L rhamnose α-L rhamnose α-L rhamnose, can solve the problems of complicated synthesis process and achieve simplified reaction steps , easy operation and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of monosaccharide receptor 1
[0029] L-rhamnose (3.28g, 20mmol) was dissolved in 75ml of dry allyl alcohol, and boron trifluoride ether (BF 3 ·OEt 2 ) (2.8ml, 10mmol), heated to 90℃~95℃ under reflux, reacted for 5~7 hours, cooled, neutralized the reaction solution with triethylamine to pH=6.8~7.2, concentrated and then column chromatography (petroleum ether: acetic acid Ethyl ester 1:20) to obtain 13.060 g of monosaccharide acceptor, with a yield of 75%.
Embodiment 2
[0030] Example 2 Synthesis of Monosaccharide Donor 2
[0031] At 0℃, to acetic anhydride (AC 2 O) (9.35ml, 100mmol) was added dropwise perchloric acid (81μL, 1mmol) to the solution. After stirring for half an hour at 0℃, L-rhamnose (1.64g, 10mmol) was added to the mixed solution and stirred at 0℃ After 2 hours, the reaction was quenched by adding ice water. Use CH 2 Cl 2 Extraction, organic phase with saturated NaHCO 3 The excess acid was neutralized, concentrated under reduced pressure and dissolved in DMF (3ml). Ammonium acetate (0.15g, 20mmol) was added and reacted for 12 hours. The mixture was extracted with water and ethyl acetate, and the organic phase was concentrated and separated by column chromatography (petroleum ether: ethyl acetate 3:1) to obtain monosaccharide receptor 2 (2.262 g) with a yield of 78%.
Embodiment 3
[0032] Example 3 Synthesis of Monosaccharide Donor 6
[0033] At 0℃, to acetic anhydride (AC 2 O) (9.35ml, 100mmol) was added dropwise perchloric acid (81μL, 1mmol) to the solution, after stirring at 0℃ for half an hour, glucose (1.8g, 10mmol) was added to the mixture, stirring at 0℃ for 2 hours before adding Ice water quenches the reaction. Use CH 2 Cl 2 Extraction, organic phase with saturated NaHCO 3 The excess acid was neutralized, concentrated under reduced pressure and dissolved in DMF (3ml). Ammonium acetate (0.15g, 20mmol) was added and reacted for 12 hours. Extract with a mixture of water and ethyl acetate, concentrate the organic phase, and separate by column chromatography (petroleum ether: ethyl acetate 3:1) to obtain monosaccharide receptor 6 (2.565 g) with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com