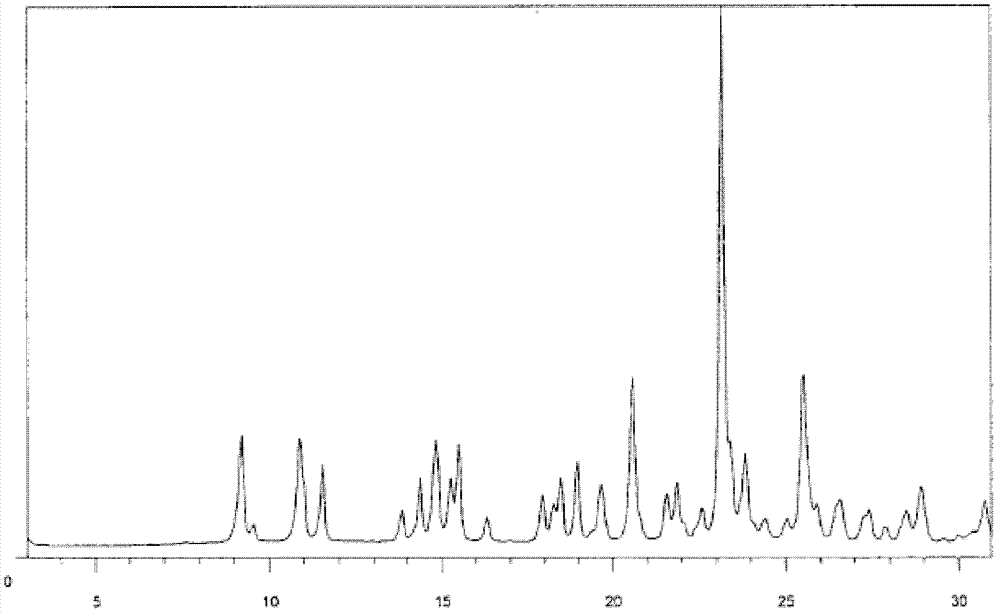
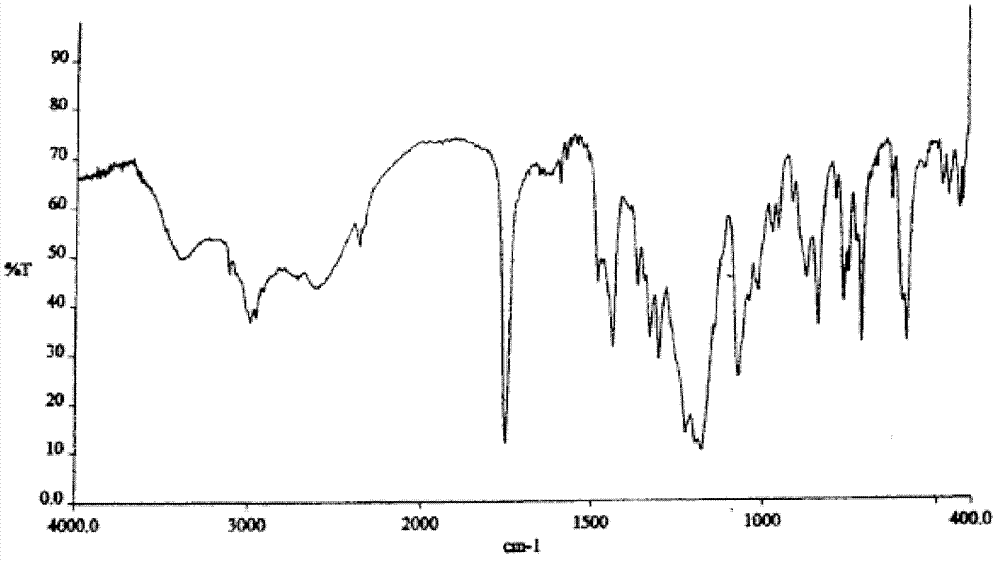
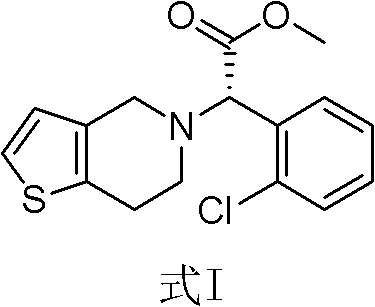
Method for preparing I-type clopidogrel hydrogen sulfate
A technology of clopidogrel hydrogen sulfate, type I, which is applied in the field of preparation of type I clopidogrel hydrogen sulfate, and can solve problems such as inability to provide stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 12.6g (0.03mol) of clopidogrel sulfate and 200mL of dichloromethane into a 500mL three-neck flask, control the room temperature, add a saturated solution of sodium bicarbonate deionized water under stirring, until the pH of the organic phase is greater than 8, and let it stand The organic phase was separated, and the aqueous phase was extracted once with 150 mL of dichloromethane. The organic phases were combined, dried and concentrated to obtain free clopidogrel base; 300 mL of isobutyl ketone was added to the free clopidogrel base, and stirred for 1 hour. It is completely dissolved; 50% stoichiometric isobutyl ketone sulfate solution is added dropwise at 30°C to make clopidogrel and sulfuric acid salt into clopidogrel bisulfate; after the dropwise addition, stir at room temperature for 12 hours; filter to obtain a solid Wash 3 times with isobutyl ketone 10mL, dry under reduced pressure at 50°C to obtain 10.5g of type I clopidogrel bisulfate, specific optical rotati...
Embodiment 2
[0024] Add 42.0g (0.1mol) of clopidogrel sulfate and 600mL of dichloromethane into a 2L three-neck flask, control the room temperature, add a saturated solution of sodium bicarbonate deionized water under stirring, until the pH of the organic phase is greater than 8, and let it stand Separate the organic phase, and extract the aqueous phase once with 500 mL of dichloromethane, combine the organic phases, dry and concentrate to obtain free clopidogrel base; add 1 L of isobutyl ketone to the free clopidogrel base, and stir for 1 hour to make It is completely dissolved; 50% stoichiometric isobutyl ketone sulfate solution is added dropwise at 30°C to make clopidogrel and sulfuric acid salt into clopidogrel bisulfate; after the dropwise addition, stir at room temperature for 12 hours; filter to obtain a solid Washed 3 times with 50 mL of isobutyl ketone, dried under reduced pressure at 50° C. to obtain 35.7 g of type I clopidogrel hydrogen sulfate, specific optical rotation: 52.0° (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com