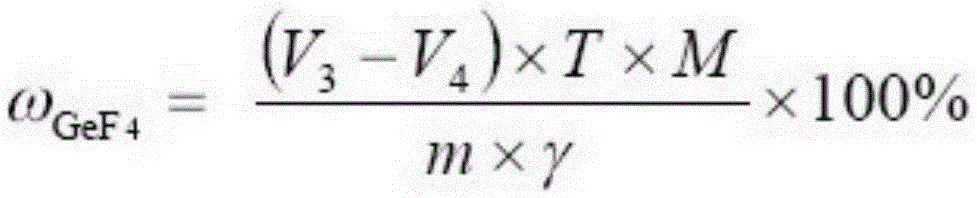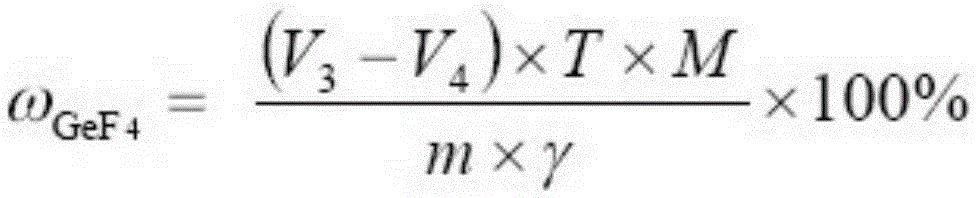Testing method for purity of germanium tetrafluoride product
A technology of germanium tetrafluoride and product purity, which is applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, and can solve the problem of no enterprise standards, industry standards or other standards, four Germanium fluoride has not established a unified standard, etc., to achieve the effect of easy operation and high measurement accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (i) Sample preparation
[0034] a. 0.6853g germanium tetrafluoride is taken out from the sample bottle by a sampling device;
[0035] b. Quantitatively absorb the sample with 20mL of 1g / ml sodium hydroxide absorption solution;
[0036] c. Transfer the solution into a beaker, add a sulfuric acid solution with a volume ratio of sulfuric acid to water of 1:1 to neutralize until the solution is slightly acidic;
[0037] d. Transfer the solution obtained in step c to a volumetric flask, and dilute to 100mL with water to obtain the sample solution;
[0038] (ii) Purity analysis of germanium tetrafluoride product
[0039] Utilize potassium iodate volumetric method to carry out titration analysis to germanium tetrafluoride sample solution, get step (i) gained sample solution 5mL in Erlenmeyer flask, add 10mL water, 3mL massfraction is the hydrochloric acid of 37%, 10mL massfraction is 85% phosphoric acid, 3g sodium hypophosphite, seal the conical flask with a cover funnel, t...
Embodiment 2
[0041] (i) Sample preparation
[0042] a. 4.2102g germanium tetrafluoride is taken out from the sample bottle by a sampling device;
[0043] b. Quantitatively absorb the sample with 40mL of 1g / ml sodium hydroxide absorption solution;
[0044] c. Transfer the solution into a beaker, add a sulfuric acid solution with a volume ratio of sulfuric acid to water of 1:1 to neutralize until the solution is slightly acidic;
[0045] d. Transfer the solution obtained in step c to a volumetric flask, and dilute to 500mL with water to obtain the sample solution;
[0046] (ii) Purity analysis of germanium tetrafluoride product
[0047] Utilize potassium iodate volumetric method to carry out titration analysis to germanium tetrafluoride sample solution, get step (i) gained sample solution 50mL in Erlenmeyer flask, add 10mL water, 6mL massfraction is the hydrochloric acid of 37%, 20mL massfraction is 85% phosphoric acid, 4g sodium hypophosphite, seal the conical flask with a cover funnel, ...
Embodiment 3
[0049] (i) Sample preparation
[0050] a. 7.8014g germanium tetrafluoride is taken out from the sample bottle by a sampling device;
[0051] b. Quantitatively absorb the sample with 40mL of 1g / ml sodium hydroxide absorption solution;
[0052]c. Transfer the solution into a beaker, add a sulfuric acid solution with a volume ratio of sulfuric acid to water of 1:1 to neutralize until the solution is slightly acidic;
[0053] d. Transfer the solution obtained in step c to a volumetric flask, and dilute to 500mL with water to obtain the sample solution;
[0054] (ii) Purity analysis of germanium tetrafluoride product
[0055] Utilize potassium iodate volumetric method to carry out titration analysis to germanium tetrafluoride sample solution, get step (i) gained sample solution 50mL in conical flask, add 10mL water, 10mL mass fraction are 37% hydrochloric acid, 40mL mass fraction is 85% phosphoric acid, 6g sodium hypophosphite, use a cover funnel to seal the conical flask, then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com