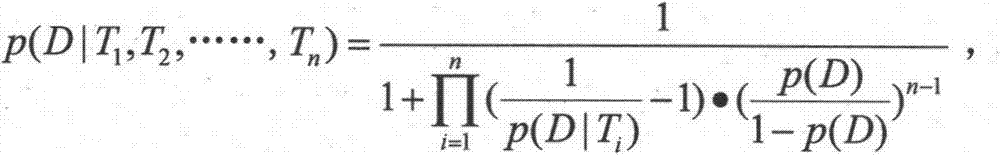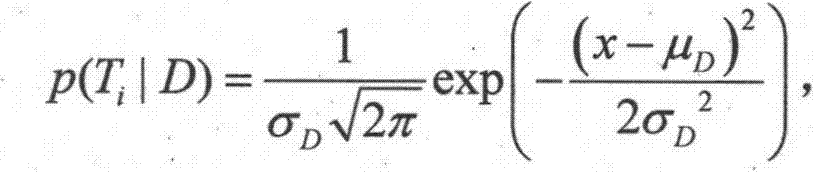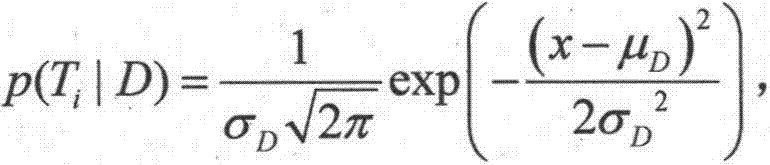Patient adverse drug reaction warning method and system based on Naive Bayes
A technology for adverse reactions and drugs, applied in special data processing applications, instruments, electrical digital data processing, etc., can solve problems such as human factor interference, and achieve the effect of improving the level of medical services
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] In order to make the present invention more comprehensible, preferred embodiments are described in detail as follows.
[0017] The invention provides a naive Bayesian-based early warning method for adverse drug reactions of patients, the steps of which are:
[0018] Step 1. Import the drug information data and adverse drug reaction case report data in the hospital's existing information system into the database when the system is initialized;
[0019] Step 2. Collect the characteristic information of the target patient: collect the current characteristic information of the target patient from the existing information system of the hospital, including age, gender, history of current illness, various clinical symptoms and examination results, etc. 1 ,T 2 ,..., T n express;
[0020] Step 3. Analyze the target patient’s medication information and related drug information: find out the target patient’s historical medication information from the hospital’s existing infor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com