Montelukast sodium chewable tablet and preparation method thereof
A technology of montelukast sodium and chewable tablets, which is applied in the field of chewable tablets containing montelukast sodium and its preparation, can solve the problems not related to the dissolution of montelukast sodium chewable tablets, and achieve uniform and bright appearance , quality controllable, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The composition of the prescription is the same as that of the reference example.
[0046] The specific preparation method is the same as that of the reference example, except that the adhesive preparation process is changed to medicinal anhydrous ethanol.
Embodiment 2
[0048] Determination of disintegration time
[0049] Take reference example, embodiment 1 and 6 samples of Singulair (batch number: 100101) on the market, and measure the disintegration of montelukast sodium chewable tablets with reference to the disintegration time limit test method (Chinese Pharmacopoeia 2010 edition two appendix XA). solution time.
[0050] Table 1 Disintegration time test results
[0051]
[0052] It can be seen from Table 1 that the sample using 95% ethanol as solvent has a longer disintegration time.
Embodiment 3
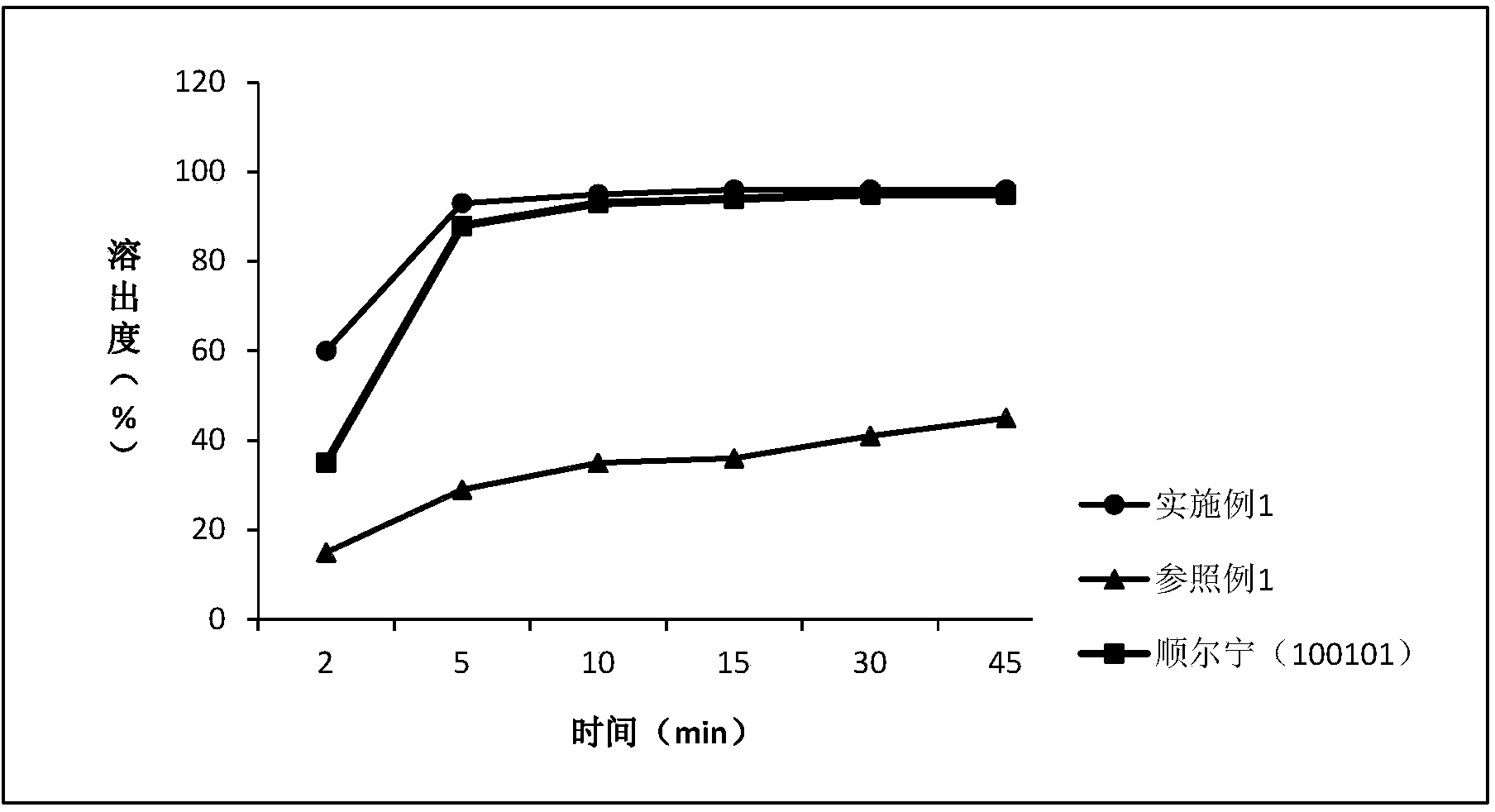
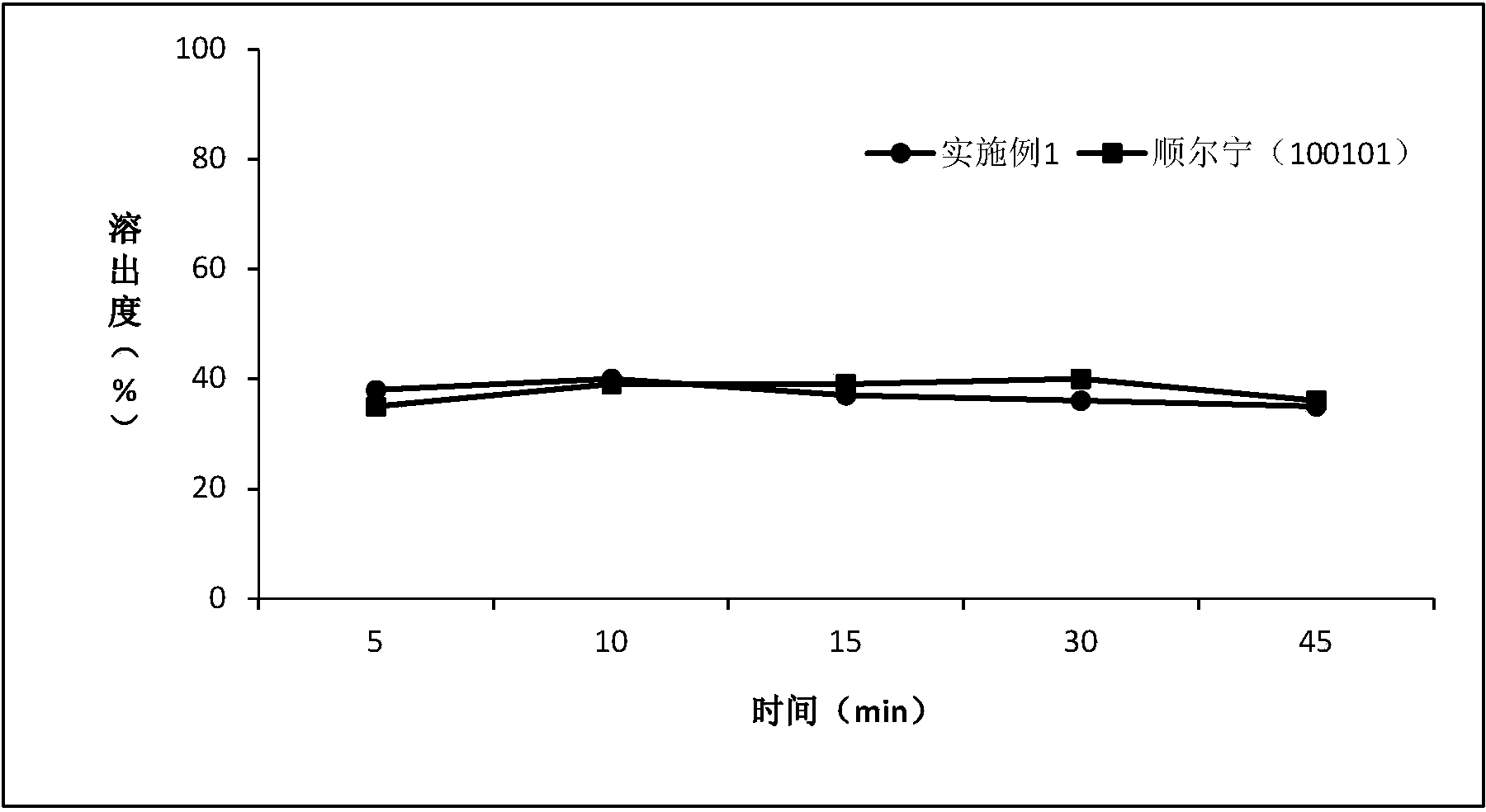
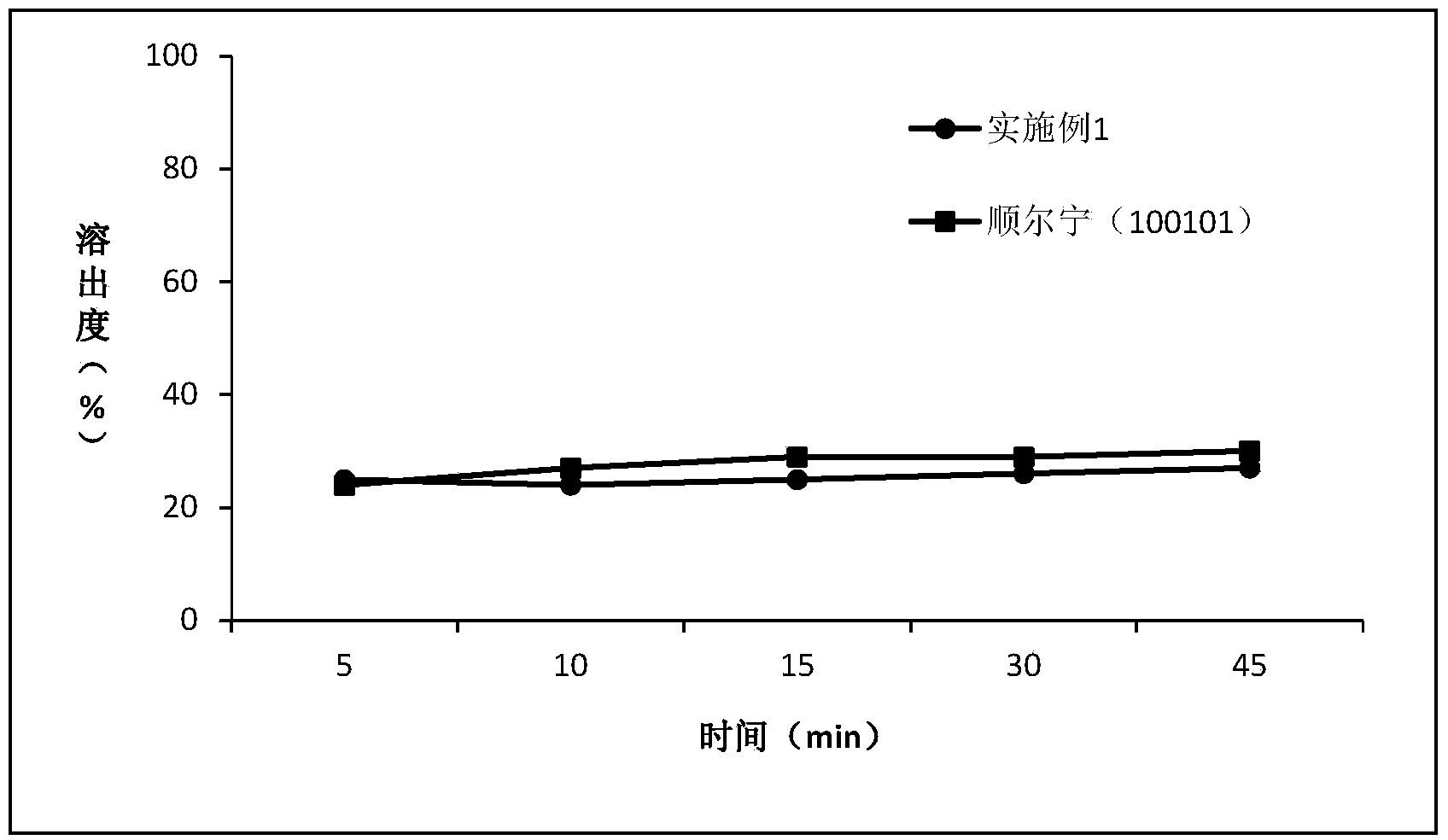
[0054] Determination of dissolution
[0055] Avoid light operation. Take this product, according to the dissolution test method (Chinese Pharmacopoeia 2010 edition two appendix XC third method), with 0.5% sodium lauryl sulfate aqueous solution, water, 0.1mol / L hydrochloric acid solution and phosphate buffer of pH6.8 200ml of solution is the dissolution medium, and the rotating speed is 100 revolutions per minute. Operate according to the law. Take an appropriate amount of solution at 2, 5, 10, 15, 30, and 45 minutes respectively, filter, and take the subsequent filtrate as the solution for the test; An appropriate amount of the reference substance of surstatin sodium was accurately weighed, dissolved in the mobile phase and quantitatively diluted to make a solution containing 20 μg per 1 ml as the reference substance solution. Measure according to the method under the content determination item, respectively take 6 tablets of each sample of the reference example and Example 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com