A kind of cathode catalyst for fuel cell and preparation method thereof
A cathode catalyst, fuel cell technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
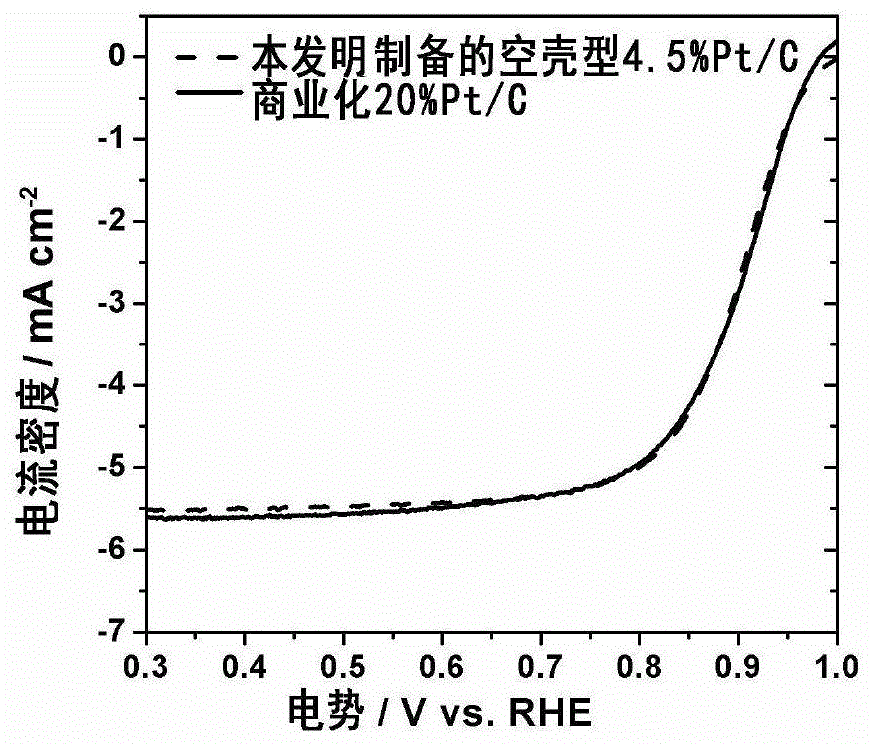
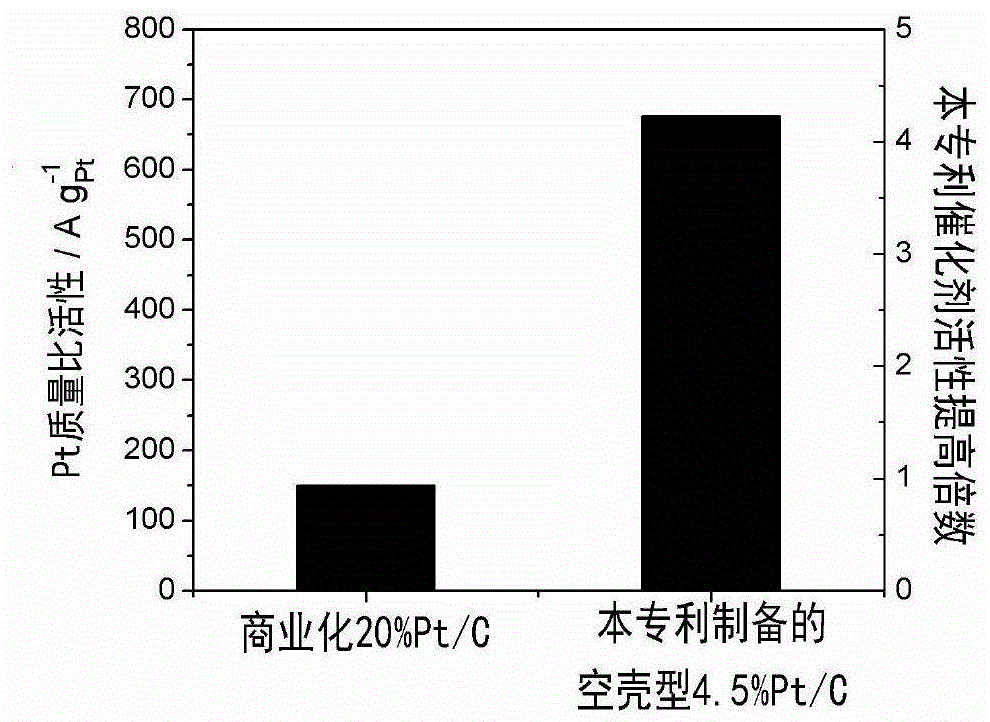
[0037] Weigh 27mgCuCl respectively 2 2H 2 O and 467mg of hexadecyltrimethylammonium bromide were dissolved in 20ml of deionized water to obtain solution A; high-purity nitrogen gas was introduced into A to saturate for 30 minutes, 120mg of XC72 carbon carrier was added, ultrasonically mixed evenly, and added under vigorous stirring 108 mg KBH 4 , to obtain slurry B; 1ml of PtCl with a concentration of 5.6mgPt / ml 4 Mix the aqueous solution and 117mg of cetyltrimethylammonium bromide in 10ml of deionized water, and stir evenly to obtain solution C; add solution C to the slurry B aged for 4 hours under stirring, and react for 4 hours under the protection of high-purity nitrogen gas; Then add 20ml of isopropanol and stir for 4 hours to obtain slurry D; centrifugally wash slurry D, vacuum dry at 60°C for 10 hours, and grind to obtain solid powder E; 2 / N 2 (Volume ratio 1:5) After the atmosphere was heat treated at 300 degrees for 5 hours, it was treated in a perchloric acid so...
Embodiment 2
[0039] Weigh 54mgCuCl respectively 2 2H 2 O and 1.58g of dodecyltrimethylammonium bromide were dissolved in 40ml of deionized water to obtain solution A; high-purity nitrogen gas was introduced into A for saturation for 30 minutes, 30mg of KB300 carbon carrier was added, ultrasonically mixed evenly, and stirred vigorously Add 250mgKBH 4 , to obtain slurry B; 4.5ml concentration of 5.6mgPt / ml K 2 PtCl 4 Mix the aqueous solution and 395mg of dodecyltrimethylammonium bromide in 10ml of deionized water, and stir evenly to obtain solution C; add solution C to the slurry B aged for 5 hours under stirring, and react for 5 hours under the protection of high-purity nitrogen gas; Then add 30ml of isopropanol, stir for 3 hours to obtain slurry D; centrifugally wash slurry D, dry in vacuum at 60°C for 6 hours, grind to obtain solid powder E; treat in a hydrochloric acid solution with a pH of 1 for 4 hours, filter and wash, After vacuum drying at 60°C for 6 hours, a shell-type Pt / C cat...
Embodiment 3
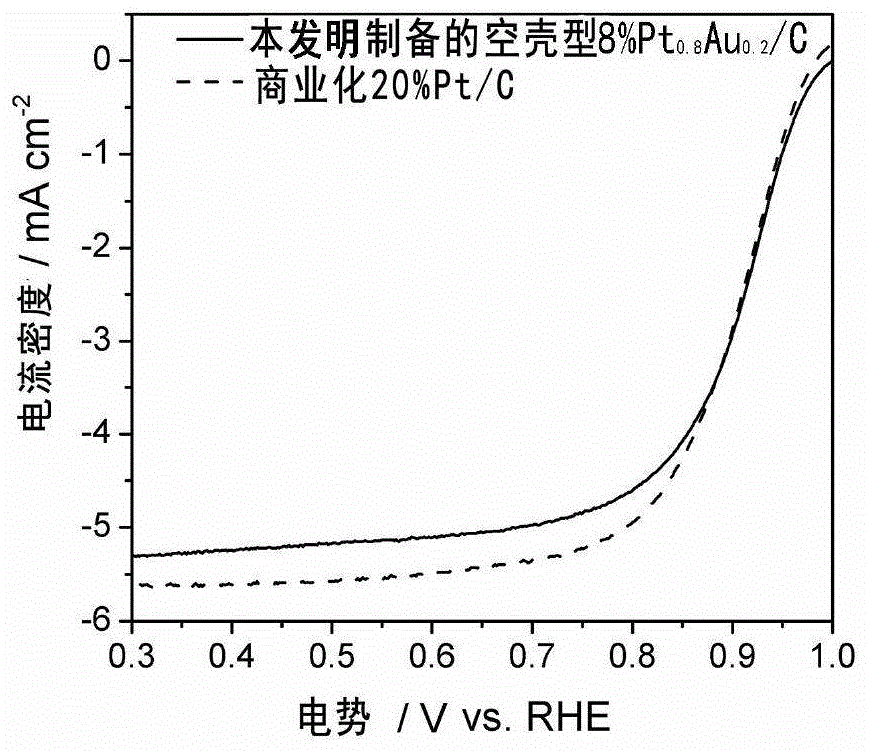
[0041] Weigh 27mgCuCl respectively 2 2H 2 O and 467mg of hexadecyltrimethylammonium bromide were dissolved in 20ml of deionized water to obtain solution A; high-purity argon gas was passed into A for saturation for 30 minutes, 60mg of XC72 carbon carrier was added, ultrasonically mixed evenly, and added under vigorous stirring 34mgNaBH 4 , to obtain slurry B; 1ml of PtCl with a concentration of 5.6mgPt / ml 4 aqueous solution and 1ml of K at a concentration of 2mgPd / ml 2 PdCl 6 The aqueous solution was mixed in 10ml of deionized water, and stirred evenly to obtain solution C; under stirring, solution C was added to the slurry B aged for 4 hours, and reacted for 4 hours under the protection of high-purity argon; then 20ml of isopropanol was added, and the slurry was obtained after stirring for 4 hours Material D; slurry D was centrifugally washed, dried in vacuum at 60 degrees for 10 hours, and ground to obtain solid powder E; solid powder E was 2 / Ar (volume ratio 1:4) atmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com