Internal reference substance for detecting bladder cancer serum miRNA and its detection primers and use
A technology for detecting primers and bladder cancer. It is applied in the directions of DNA/RNA fragments, recombinant DNA technology, and microbial measurement/inspection. It can solve problems such as lack of internal reference molecules, inconsistent internal references, and restrictions on the transformation of research results. Application prospects, elimination of expression level differences, stable and reliable detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Collection of Specimen and Data Arrangement
[0044] The inventors of the present invention collected serum samples from 250 bladder cancer patients from Qilu Hospital of Shandong University from 2011 to 2013, and collected serum from 158 control subjects during the same period, which were used as experimental samples for Solexa sequencing and subsequent qRT-PCR screening and verification. .
[0045] (1) 250 patients with bladder cancer diagnosed by pathology had not undergone surgery, radiotherapy and chemotherapy before blood collection.
[0046] (2) A total of 158 healthy male and female controls were included.
[0047] The demographic data and clinicopathological data of the above subjects were systematically collected.
Embodiment 2
[0048] Example 2 Solexa sequencing of serum miRNA
[0049] The samples selected in the sequencing stage included 10 invasive bladder cancers, 10 non-invasive bladder cancers, and 10 controls. The three groups of samples were sequenced by Solexa to obtain relevant results (the kits were purchased from ABI). The specific steps are:
[0050] (1) Take 15ml of serum from each group of samples, add an equal volume of Trizol reagent and mix thoroughly;
[0051] (2) Place at room temperature for 30 minutes, then add chloroform at a volume ratio of 0.2 ml of chloroform per 1 ml of Trizol reagent, shake vigorously for 10 s, 20 minutes at room temperature, 12,000 g, 4 °C, and centrifuge for 15 minutes;
[0052] (3) Carefully transfer the supernatant to a new centrifuge tube, and use the 3-step phenol / chloroform method to remove the protein;
[0053] (4) Transfer the aqueous phase to a new centrifuge tube, then add isopropanol at a volume ratio of 0.5 ml of isopropanol per 1 ml of Trizo...
Embodiment 3
[0062] Example 3 qRT-PCR screening and verification of serum miRNA
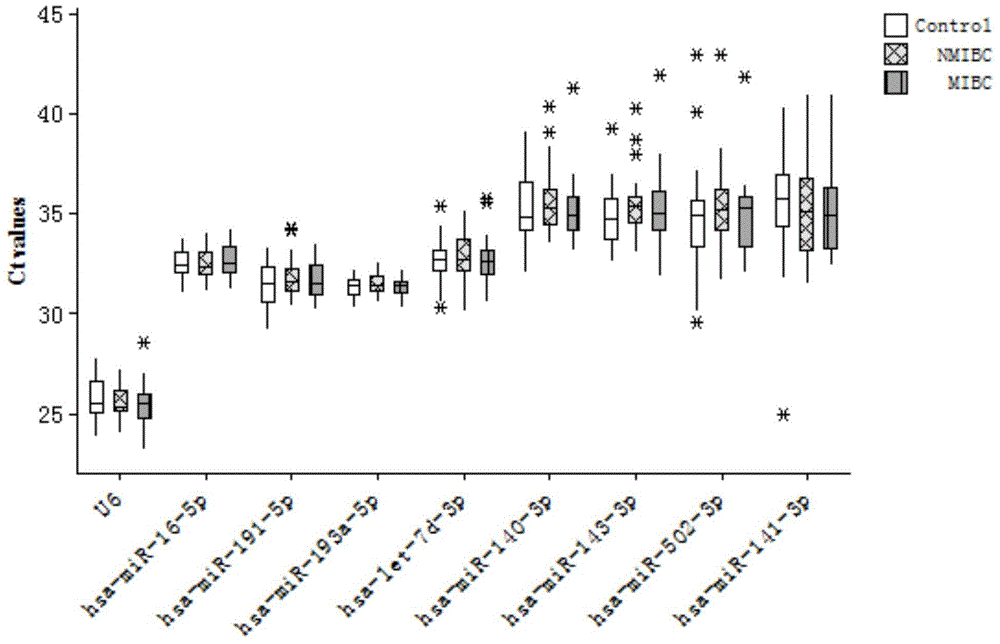
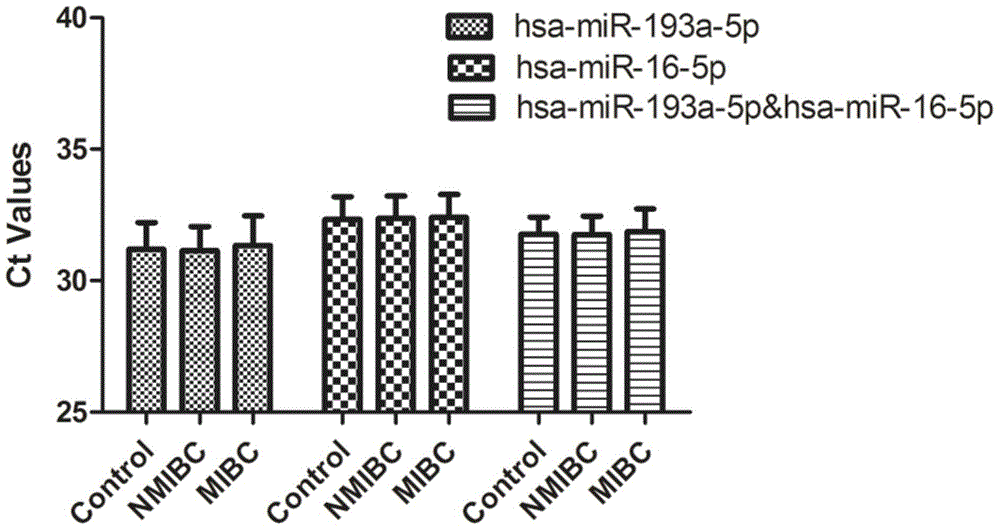
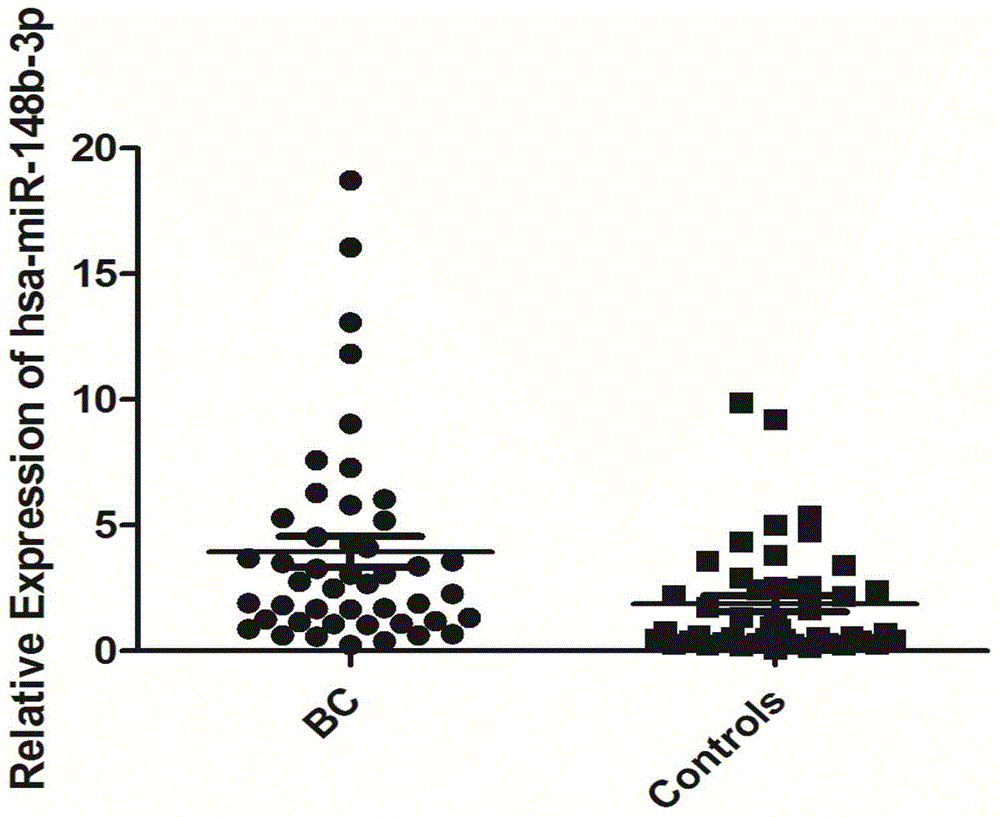
[0063] According to Solexa sequencing results, miRNA molecules meeting the following criteria were selected for further screening using qRT-PCR technology: a) The expression copy number was greater than 50 in invasive bladder cancer, non-invasive bladder cancer and the control group; b) In the three groups All were stably expressed, and there was no significant difference between the three groups (p≥0.05). According to the above criteria, a total of 10 miRNA molecules that meet the requirements (including hsa-miR-193a-5p, hsa-miR-191-5p, hsa-miR-16-5p, hsa-miR-10a-5p, hsa- miR-345-5p, hsa-miR-143-3p, hsa-miR-140-3p, hsa-miR-502-3p, let-7d-3p, hsa-miR-141-3p). In addition, since U6 is often used as an internal reference molecule for tissue miRNA detection, in order to verify whether it can be used as an internal reference in serum, U6 was also included in the screening as a candidate molecule. The above 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com