Internal reference substance for detecting bladder cancer serum miRNA and its detection primers and use
A technology for the detection of primers and bladder cancer, which is applied in DNA/RNA fragments, recombinant DNA technology, and the determination/examination of microorganisms. Stable and reliable performance, broad application prospects, and the effect of eliminating differences in expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Collection of Specimens and Data Arrangement
[0044] The inventors of the present invention collected serum samples from 250 patients with bladder cancer from Qilu Hospital of Shandong University between 2011 and 2013, and collected serum samples from 158 controls during the same period, which were used as experimental samples for Solexa sequencing and subsequent qRT-PCR screening and verification .
[0045] (1) 250 patients with bladder cancer diagnosed by pathology, all of whom had not undergone surgery, radiotherapy and chemotherapy before blood collection.
[0046] (2) A total of 158 healthy male and female controls.
[0047] The system collected the demographic data, clinicopathological data and other information of the above subjects.
Embodiment 2
[0048] Example 2 Solexa sequencing of serum miRNA
[0049] The samples selected in the sequencing stage included 10 cases of invasive bladder cancer, 10 cases of non-invasive bladder cancer and 10 controls. The three groups of samples were sequenced by Solexa to obtain relevant results (the kit was purchased from ABI). The specific steps are:
[0050] (1) Take 15ml of serum for each group of samples, add an equal volume of Trizol reagent and mix well;
[0051] (2) Stand at room temperature for 30 minutes, then add chloroform at a volume ratio of 0.2ml chloroform per 1ml Trizol reagent, shake vigorously for 10s, room temperature for 20 minutes, centrifuge at 12,000g, 4°C for 15 minutes;
[0052] (3) Carefully transfer the supernatant to a new centrifuge tube, and use the 3-step phenol / chloroform method to remove protein;
[0053] (4) Transfer the water phase to a new centrifuge tube, then add isopropanol at a volume ratio of 0.5ml isopropanol per 1ml Trizol reagent, let stand...
Embodiment 3
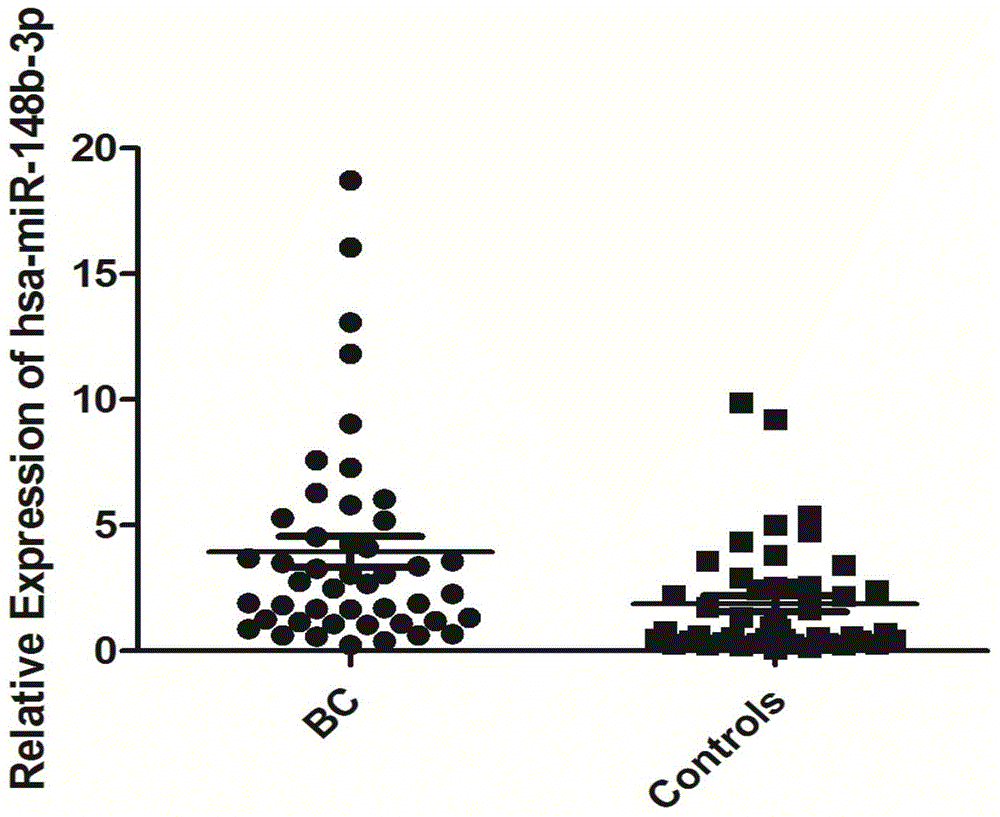
[0062] Example 3 qRT-PCR screening and verification of serum miRNA
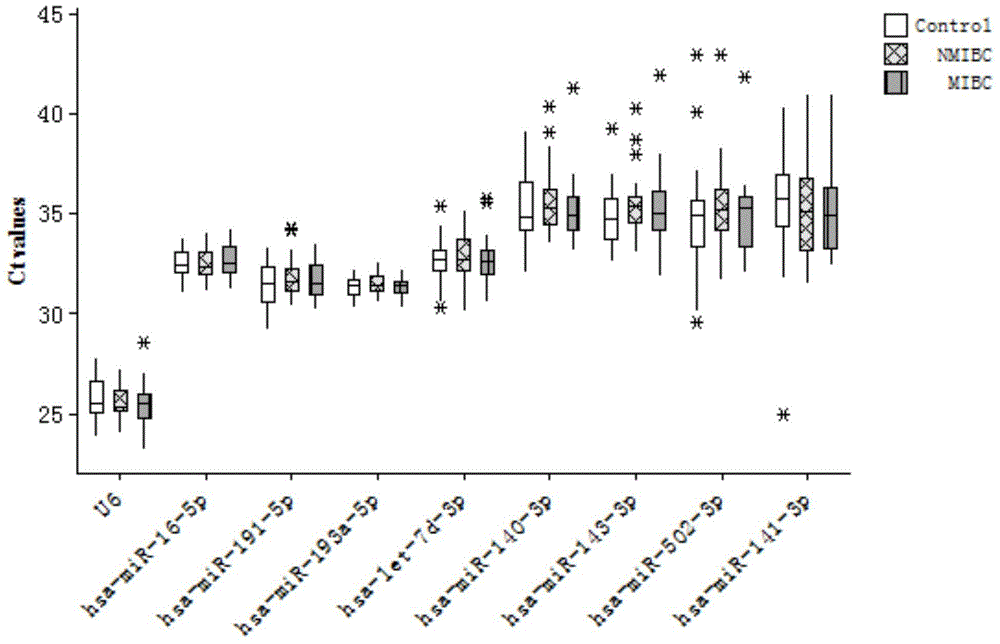
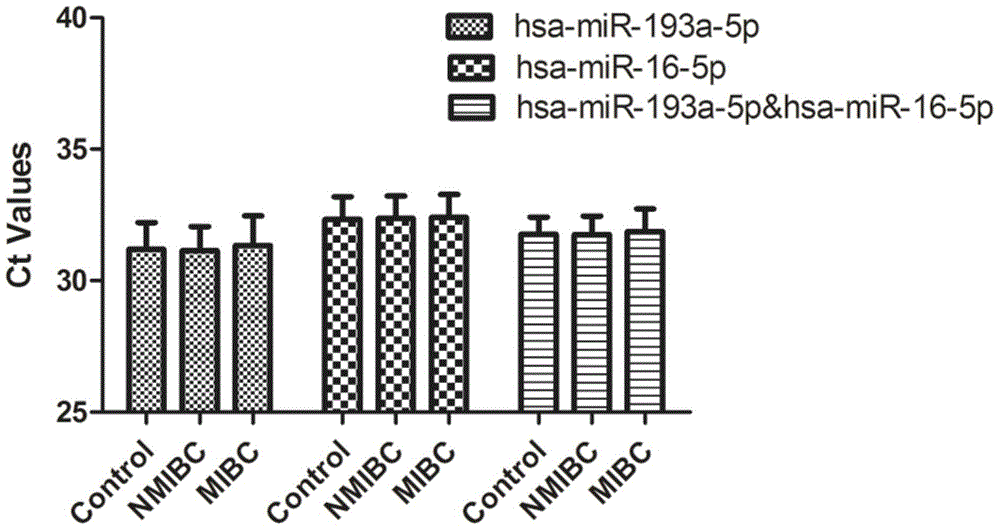
[0063] According to the results of Solexa sequencing, select miRNA molecules that meet the following criteria for further screening by qRT-PCR technology: a) the expression copy number is greater than 50 in invasive bladder cancer, non-invasive bladder cancer and the control group; b) in the three groups All were stably expressed, and there was no significant difference among the three groups (p≥0.05). According to the above criteria, a total of 10 miRNA molecules meeting the requirements were selected (including hsa-miR-193a-5p, hsa-miR-191-5p, hsa-miR-16-5p, hsa-miR-10a-5p, hsa- miR-345-5p, hsa-miR-143-3p, hsa-miR-140-3p, hsa-miR-502-3p, let-7d-3p, hsa-miR-141-3p). In addition, since U6 is often used as an internal reference molecule for tissue miRNA detection, U6 was also included in the screening as a candidate molecule in order to verify whether it can be used as an internal reference in serum. The abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com