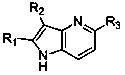
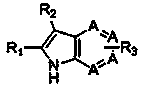
Azaindole with regionselectivity and synthetic method thereof
A regioselective, azaindole technology, applied in the field of azaindole and its synthesis, can solve the problems of low reaction yield of diethylene glycol and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 : 2-Bromo-6,7,8,9-tetrahydro-5H-pyrido[3,2,b]indole preparation of
[0069]
[0070] Steps:
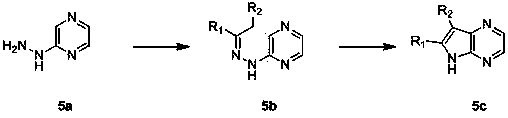
[0071] React 2-bromo-5-fluoropyridine (4 g, 23 mmol) and 10 ml of hydrazine hydrate with a microwave oven at 110 ° C for 1 hour. After cooling, pour the reaction solution into 60 ml of water to precipitate a solid, filter, and dry the filter cake to obtain 2 grams of 2-bromo-5-hydrazine pyridine, yield 47%.
[0072] 1 H NMR (400MHz, CDCl 3 ) d : 7.99 (br. s., 1H), 7.30 (s, 1H), 7.11 (d, J =8.0 Hz, 1H), 5.28 (s, 1H), 3.64 (s, 2H).
[0073] 2-bromo-5-hydrazine pyridine (2 g, 11 mmol) and cyclohexyl ketone (1.15 g, 12 mmol) were reacted in 15 ml of ethanol to water four to one at room temperature for 1 hour, and the solid was filtered to obtain 0.77 Gram 2-bromo-5-cyclohexylhydrazonepyridine, yield 28%.
[0074] 1 H NMR (400MHz, CDCl 3 ) d : 8.07 (s, 1H), 7.39 - 7.33 (m, 1H), 7.33 - 7.29 (m, 1H), 7.07 (s, 1H), 2.37 - 2.31 (m, 4H), 1.91 - 1.99 (m, 2H) , 1...
Embodiment 2
[0077] Example 2 : 5-Bromo-2,3-dimethyl-1H-pyrrolo[3,2,b]pyridine preparation of
[0078]
[0079] Steps:
[0080] 2-bromo-5-hydrazinopyridine (8 g, 46.2 mmol) and butan-2-ketone (5 g, 69.4 mmol) were reacted in 65 ml of ethanol at room temperature for 18 hours, and the reaction solution was concentrated under pressure to obtain 4.34 g of 2 - Crude bromo-5-(2-(butan-2-hydrazone)pyridine.
[0081] Put 4.34 grams of crude product 2-bromo-5-(2-(butyl-2-hydrazone)pyridine in 15 ml of heat-conducting oil, react with microwave at 250°C for 10 minutes, and put the reaction liquid directly on the silica gel column after cooling. About 20 to 1 ester was used as the eluent, and the collected fractions were concentrated under reduced pressure to obtain 2.8 g of 5-bromo-2,3-dimethyl-1H-pyrrolo[3,2,b]pyridine, with a yield of 69%.
[0082] 1 H NMR (400MHz, CDCl 3 ) δ : 7.37 (d, J =8.1 Hz, 1H), 7.14 (d, J =8.1 Hz, 1H), 2.42(s, 3H), 2.37(s, 3H).
Embodiment 3
[0083] Example 3 : 5-bromo-3-isopropyl-1H-pyrrolo[3,2,b]pyridine preparation of
[0084]
[0085] Steps:
[0086] 2-Bromo-5-hydrazine pyridine (5 g, 26.6 mmol) and 3-methylbutyraldehyde (3.44 g, 39.9 mmol) were reacted at room temperature in 50 ml of ethanol for 18 hours, and the reaction solution was concentrated under pressure to obtain 3.8 g of crude 2-bromo-5-(2-(3-methylbutylhydrazone)pyridine.
[0087] Put 3.8 grams of crude product 2-bromo-5-(2-(3-methylbutylhydrazone)pyridine in 15 ml of heat-conducting oil, react with microwave at 250°C for 10 minutes, and put the reaction liquid directly on the silica gel column after cooling, petroleum ether ratio About 20:1 ethyl acetate was used as the eluent, and the collected fractions were concentrated under reduced pressure to obtain 1.7 g of 5-bromo-3-isopropyl-1H-pyrrolo[3,2,b]pyridine, with a yield of 49%.
[0088] 1 H NMR (400MHz, CDCl 3 ) δ : 7.48 (d, J =8.1 Hz, 1H), 7.22 (s, 1H), 7.19 (d, J =8.1 Hz, 1H), 3...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap