Adverse drug reaction (ADR) report quality evaluation system and ADR report quality evaluation method
A technology for adverse reactions and quality assessment, applied in special data processing applications, instruments, electrical digital data processing, etc., can solve problems such as the inability to automatically identify the quality of report forms, increase manpower, and limited statistical analysis capabilities to improve quality Assessing capabilities, improving quality levels, and promoting quality-level effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The present invention will be further described below in conjunction with the accompanying drawings and embodiments.
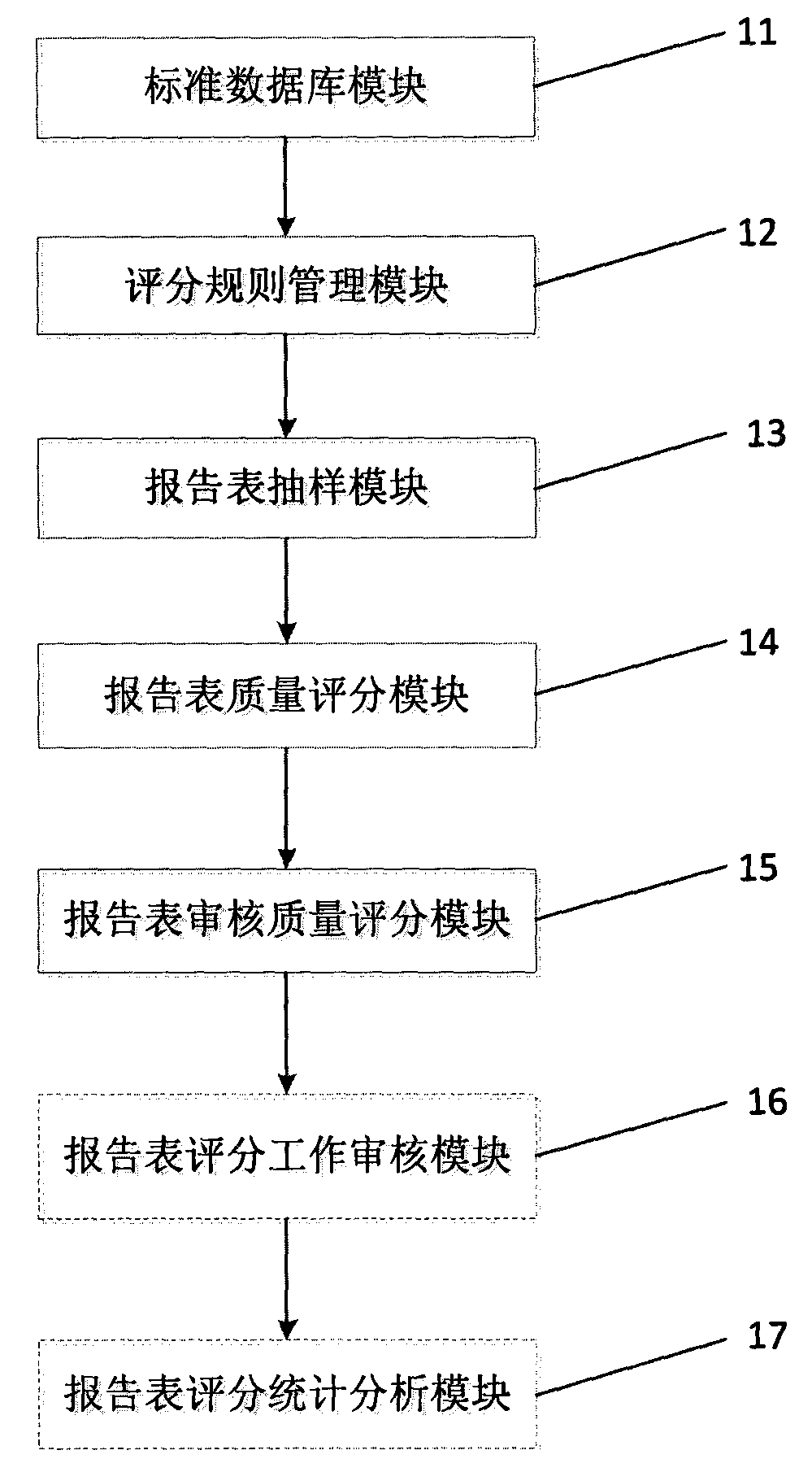
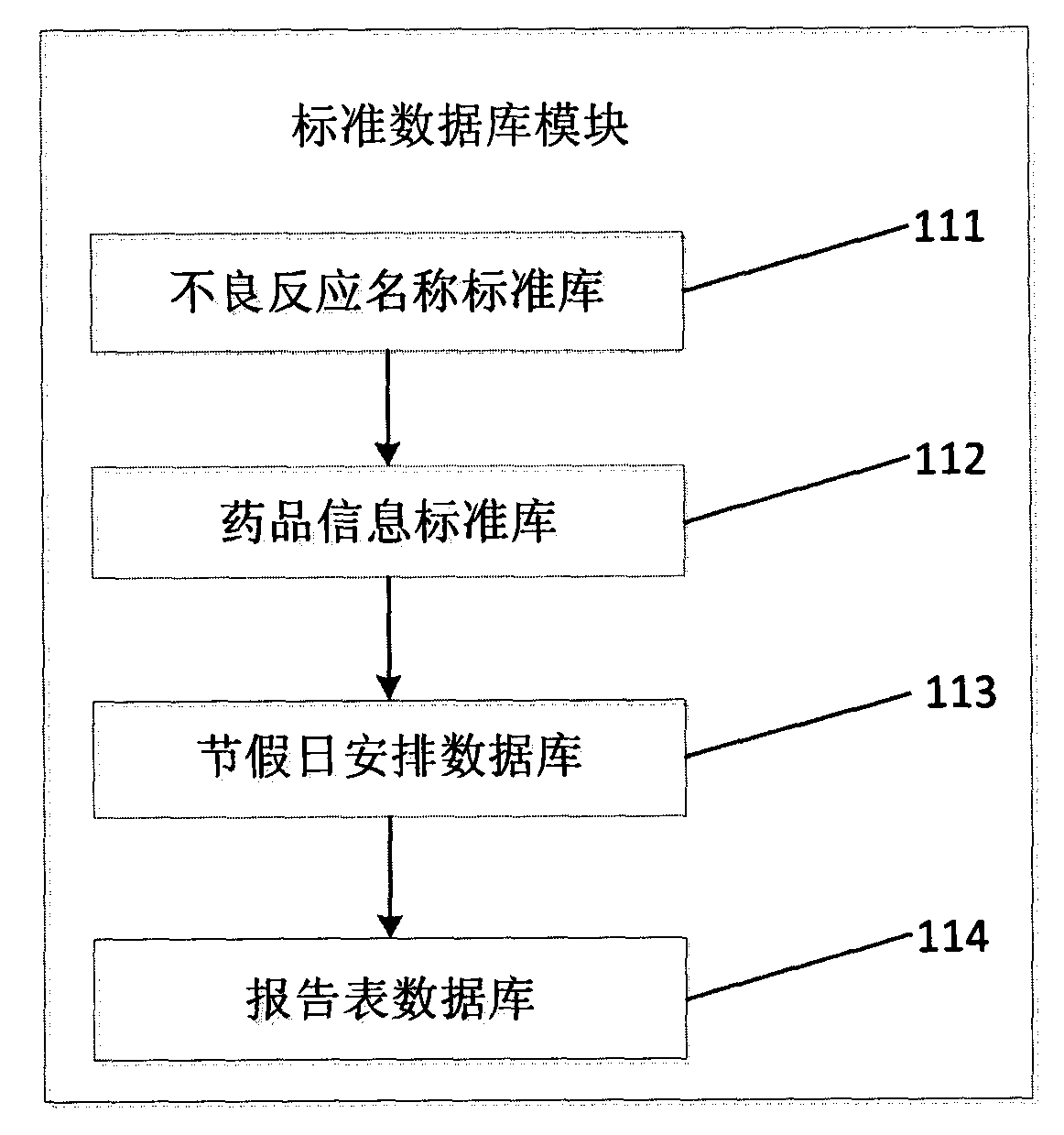
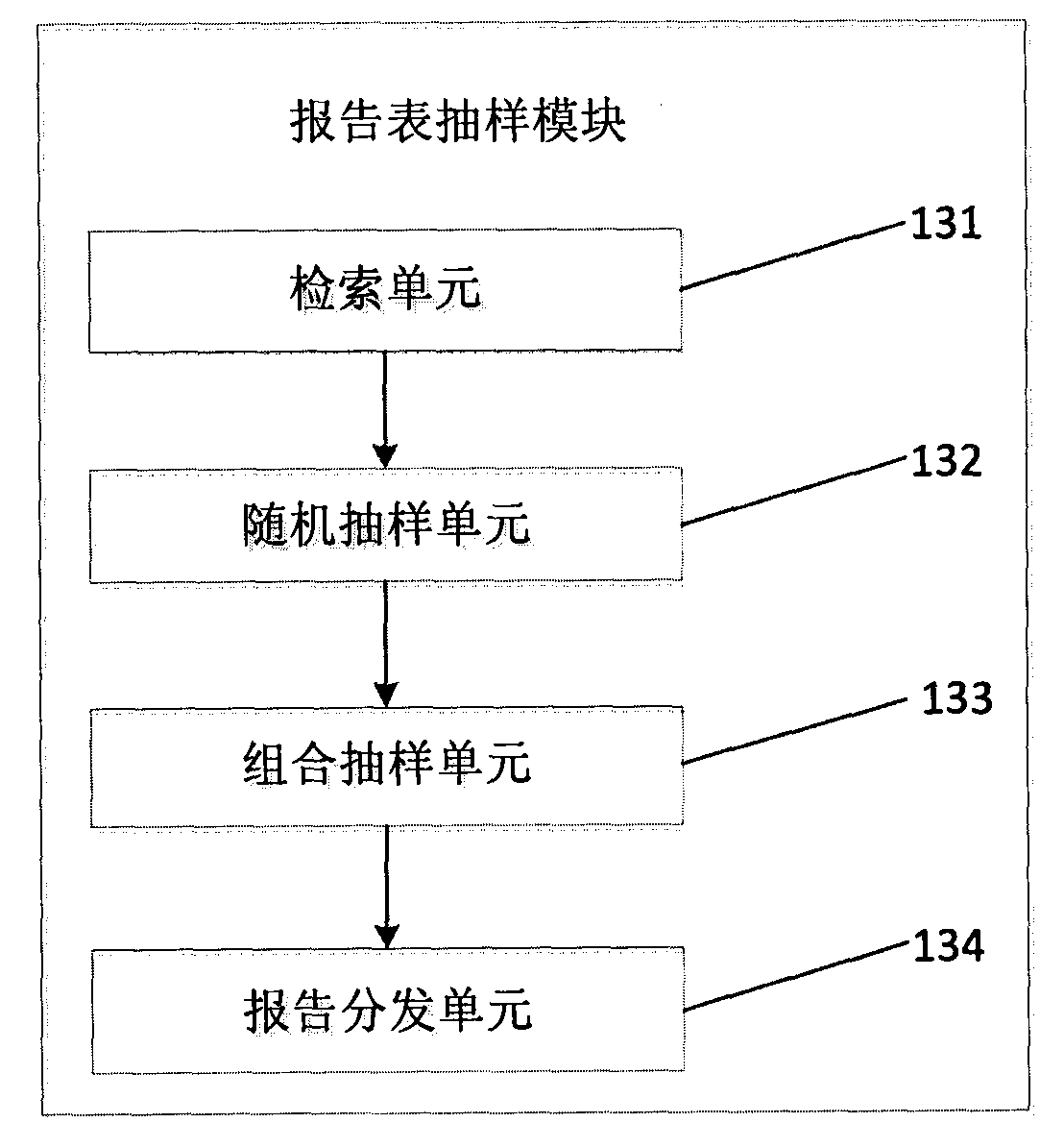
[0019] figure 1 It is a structural schematic diagram of the adverse drug reaction report quality evaluation system of the present invention; figure 2 It is a schematic structural diagram of the standard database module of the adverse drug reaction report quality assessment system of the present invention; image 3 It is a schematic structural diagram of the report form sampling module of the adverse drug reaction report quality assessment system of the present invention.
[0020] See Figure 1 ~ Figure 3 , the adverse drug reaction report quality assessment system provided by the present invention includes a standard database module 11 for recording standard data information, and also includes: a scoring rule management module 12 for setting scoring items; a report form sampling module 13 for Sampling the data; the report form quality scoring module...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com