Hydroxyphenyl benzyl ether derivative as well as preparation method and application thereof
A technology of phenylbenzyl ether and its derivatives, applied in the field of pharmaceutical compounds, can solve the problems of high fat solubility, poor stability in vivo, slow brain clearance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
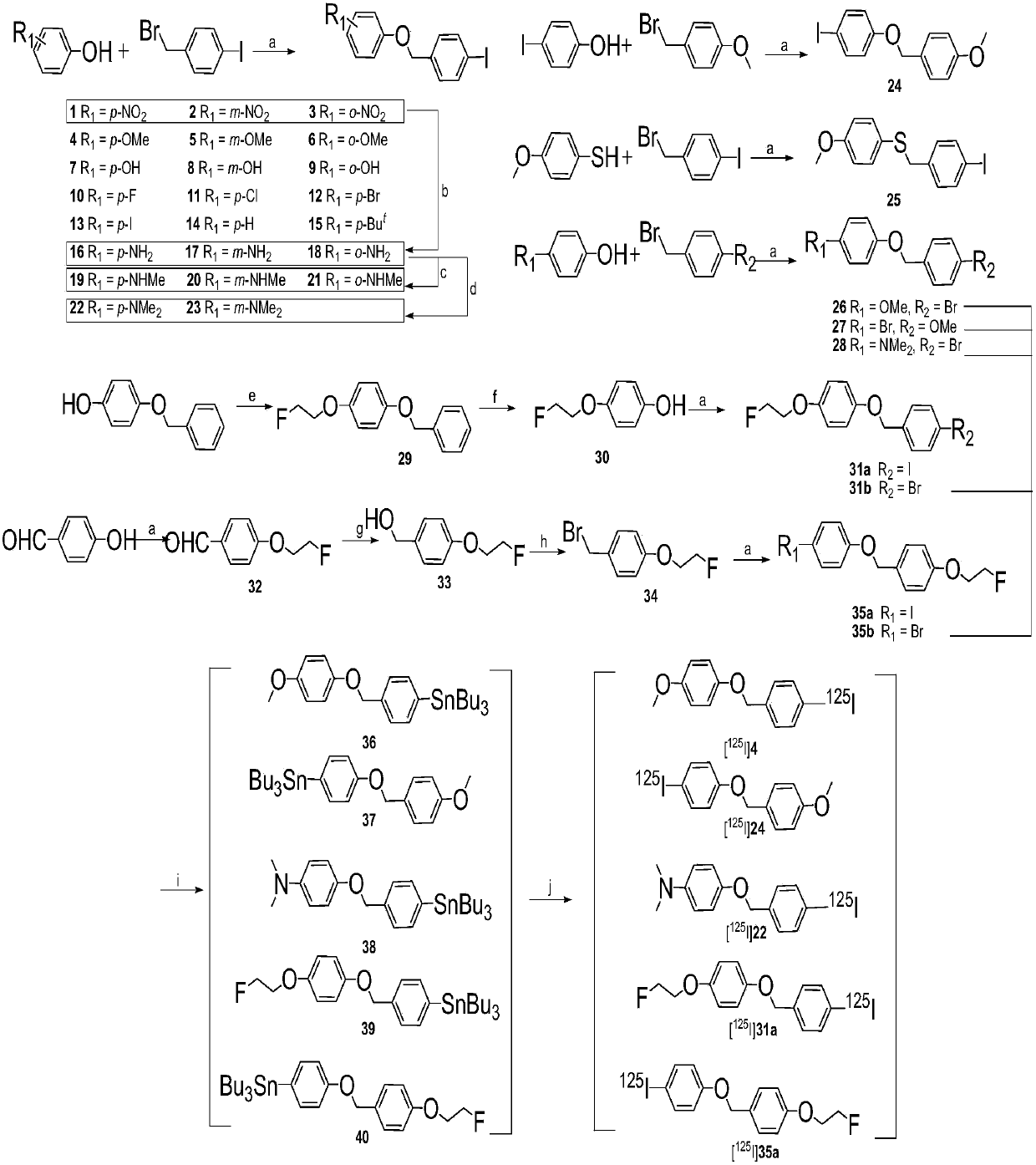
[0125] Embodiment 1: the synthesis of iodo compound
[0126] The synthetic reaction scheme is as follows figure 1 As shown, the compound numbers in this example are all consistent with the numbers in the reaction scheme of this figure.
[0127] exist figure 1 In the synthetic route shown, reagent and condition are as follows: (a) K 2 CO 3 , DMF, 90℃; (b) SnCl 2 2H 2 O, EtOH, HCl, reflux; (c) 1: NaOMe, (CH 2 O) n , MeOH, reflux; 2: NaBH 4 , reflux; (d) (CH 2 O) n ,NaBH 3 CN, HAc, r.t.; (e) 1-bromo-2-fluoroethane, KOH, ethanol, reflux; (f) 10%Pd / C, 1atm H 2 ,50℃;(g)NaBH 4 ,MeOH,0℃;(h)PBr 3 ,CH 2 Cl 2 , r.t.; (i) (Bu 3 Sn) 2 ,(PPh 3 ) 4 Pd, toluene, Et 3 N, reflow; (j)[ 125 I]NaI,HCl(1M),H 2 o 2 (3%).
[0128] 1) Synthesis of 1-iodo-4-((4-nitrobenzyloxy)methyl)benzene (1-iodo-4-((4-nitrophenoxy)methyl)benzene, compound 1)
[0129] Dissolve 4-nitrophenol (2.78g, 20mmol), 4-iodobenzyl bromide (5.00g, 20mmol) in 5mL of anhydrous DMF, add K 2 CO 3 (5.53 g, ...
Embodiment 2
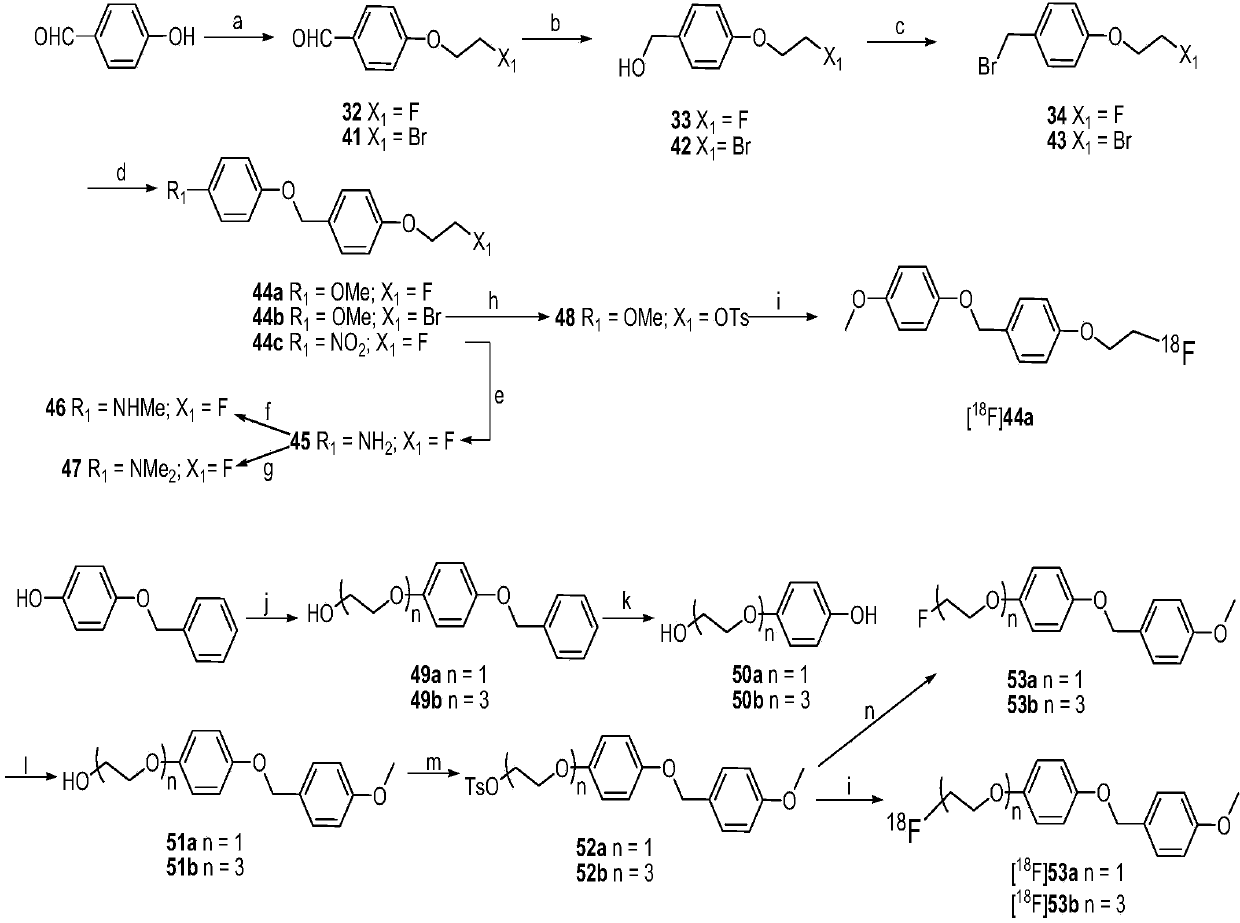
[0212] Embodiment 2: the synthesis of fluorinated compound
[0213] The synthetic reaction scheme is as follows figure 2 As shown, the compound numbers in this example are all consistent with the numbers in the reaction scheme of this figure.
[0214] exist figure 2 In the synthetic route shown, reagent and condition are as follows:
[0215] (a) 1-bromo-2-fluoroethane (1-bromo-2-fluoroethane) or1,2-dibromoethane (1,2-dibromoethane), K 2 CO 3 ,DMF,90℃;(b)NaBH 4 , MeOH, 0°C; (c) PBr 3 ,CH 2 Cl 2 , r.t.; (d) 4-methoxyphenol (4-methoxyphenol) or4-nitrophenol (4-nitrophenol), K 2 CO 3 , DMF, 90℃; (e) SnCl 2 2H 2 O, EtOH, HCl, reflux; (f) 1: NaOMe, (CH 2 O) n , MeOH, reflux; 2: NaBH 4 , reflux; (g) (CH 2 O) n ,NaBH 3 CN, HAc, r.t.; (h) AgOTs, MeCN, reflux; (i) 18 f - , K 2 CO3, Kryptofix-2.2.2 (ie K 222 , amino polyether), acetonitrile, 100 ° C; Ethoxy)ethanol instead of 2-chloroethanol), KOH, EtOH, reflux; (k) 10%Pd / C, 1atm H 2 ,50°C; (l) 1-(chloromethyl)-4...
Embodiment 3
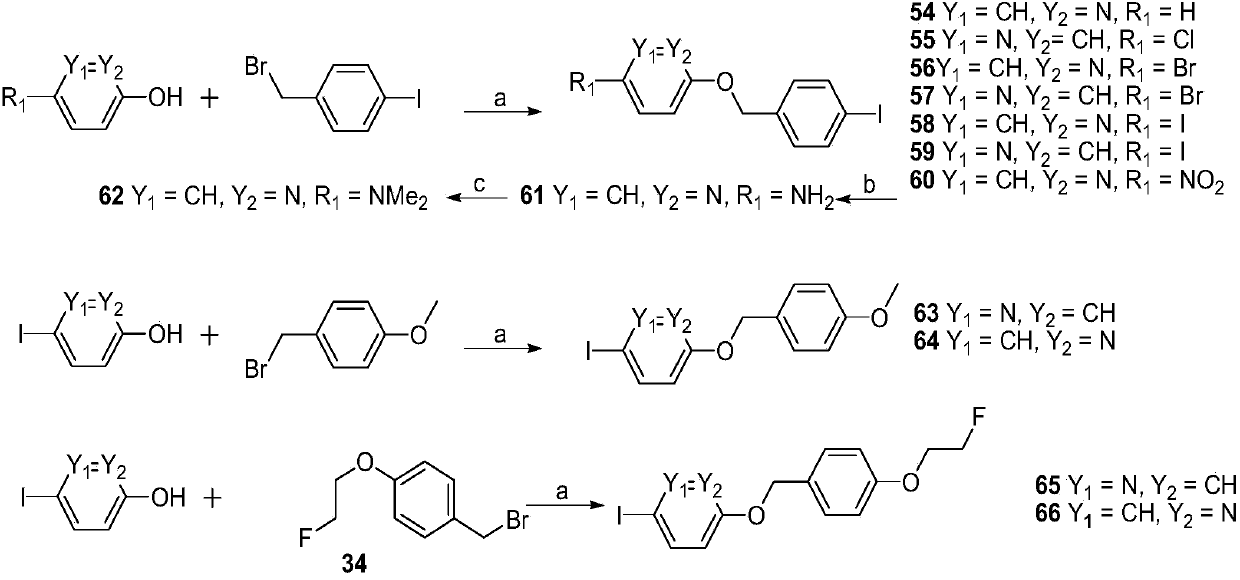
[0256] Embodiment 3: the synthesis of pyridine derivatives
[0257] The synthetic reaction scheme is as follows image 3 As shown, the compound numbers in this example are all consistent with the numbers in the reaction scheme of this figure.
[0258] exist image 3 In the synthetic route shown, reagent and condition are as follows: (a) K 2 CO 3 , DMF, 90℃; (b) SnCl 2 2H 2 O, EtOH, HCl, reflux; (c) CH 3 I,K 2 CO 3 , r.t.
[0259] 1) Synthesis of 2-(4-iodobenzyloxy)pyridine (2-((4-iodobenzyl)oxy)pyridine, compound 54)
[0260] It was prepared according to the method of compound 1 (2-hydroxypyridine was used instead of 4-nitrophenol) to obtain white solid 54 (187.5 mg, 60.3%). 1 H NMR (400MHz, CDCl 3 )δ7.67(d,J=8.2Hz,2H),7.34(ddd,J=8.8,6.7,1.8Hz,1H),7.26–7.24(m,1H),7.06(d,J=8.2Hz,3H ),6.66(d,J=9.1Hz,1H),6.19(t,J=6.7Hz,1H),5.09(s,2H).
[0261] 2) Synthesis of 2-chloro-5-(4-iodobenzyloxy)pyridine (2-chloro-5-((4-iodobenzyl)oxy)pyridine, compound 55)
[0262] It was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com