β-hinokitiol ester or salt and its application in the preparation of animal feed additives
A technology for hinokitiol ester and animal feed, which is applied in the directions of animal feed, animal feed, application, etc., can solve the problems of undisclosed salt structure, light instability, unexplained complexes, etc., and achieves good application prospects and strong growth. The effect of promoting performance and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
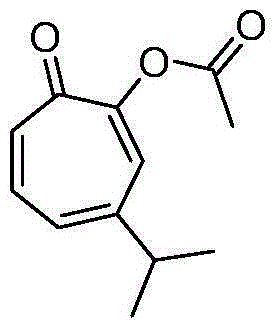
[0023] Structural formula:
[0024]
[0025] Preparation of 3-isopropyl-1,3,5-cycloheptatrien-7-one-ethyl ester
[0026] making process:
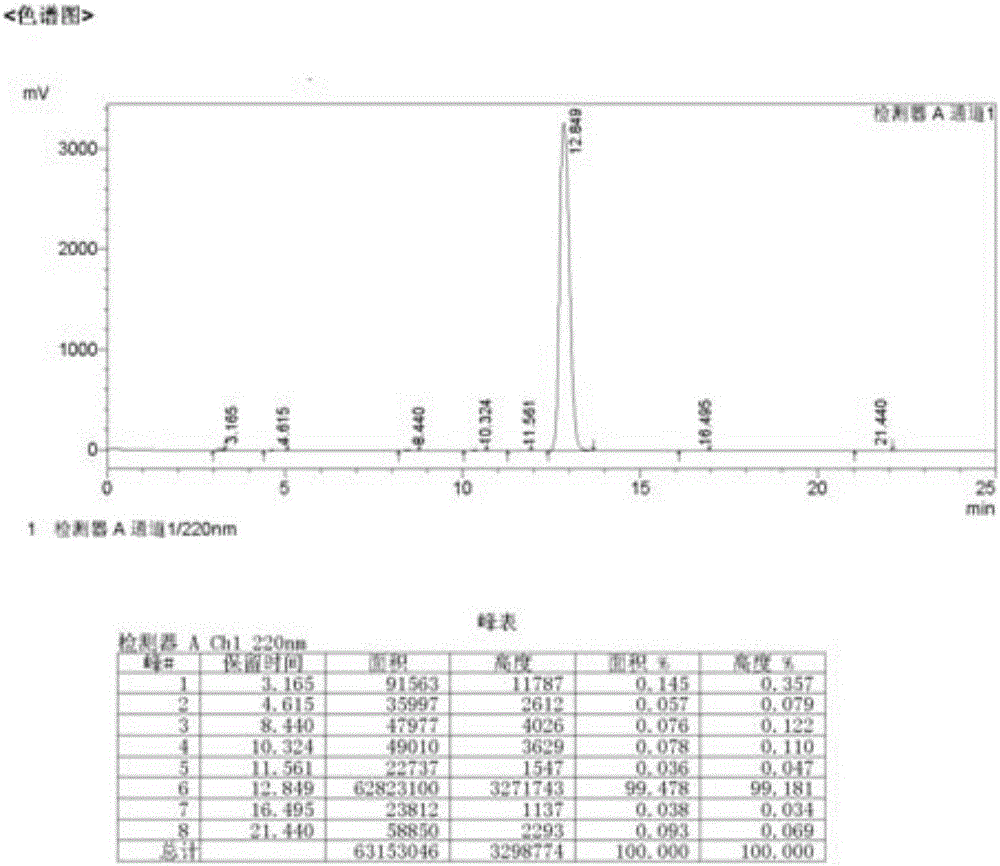
[0027] 2-Hydroxy-4-isopropyl-2,4,6-cycloheptatrien-1-one (1.64g, 10mmol, 1eq) and triethylamine (1~10eq) were dissolved in 100ml of dichloromethane,- 10℃~25℃, take acetyl chloride (0.8~5eq) and dissolve it in about 10ml of dichloromethane, slowly add it dropwise to the mixed solution, then keep stirring for 3~8 hours, TLC (developing agent petroleum ether: ethyl acetate Esters=5:1) shows that the basic reaction of the raw materials is complete, and new spots are generated. About 100ml of water was added to extract the layers, and the organic phase was washed with water once more. The solvent was removed by rotary evaporation of the organic phase to obtain a brown-red oil, which was confirmed to be the target product (3-isopropyl-1,3,5-cycloheptatrien-7-one-ethyl ester) by HNMR, with a yield of about 86%. Unstable, ester hydrolysis oc...
Embodiment 2
[0031] Structural formula:
[0032]
[0033] Preparation of 3-isopropyl-1,3,5-cycloheptatrien-7-one-n-decyl ester
[0034] making process:
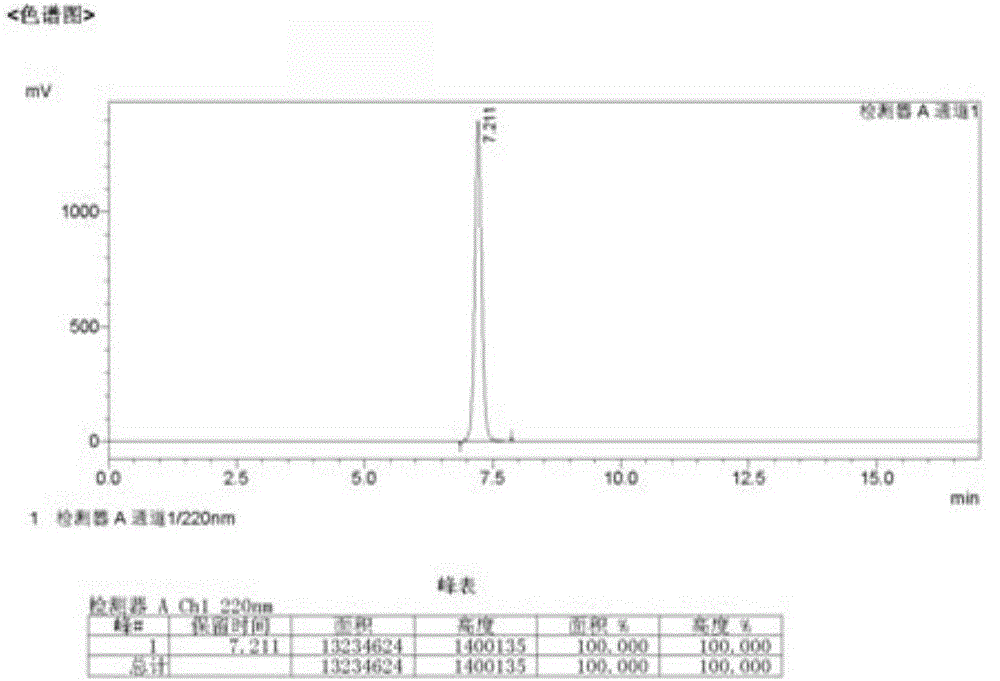
[0035] 2-Hydroxy-4-isopropyl-2,4,6-cycloheptatrien-1-one (3.28g, 20mmol, 1eq) and triethylamine (1~10eq) were dissolved in 100ml of dichloromethane,- 10°C~25°C, take n-decanoyl chloride (0.8~5eq) and dissolve it in about 10ml of dichloromethane, slowly add it dropwise to the mixed solution, then keep stirring for 3~8 hours, TLC (developer petroleum ether: acetic acid Ethyl ester=5:1) shows that a small amount of raw materials are not completely reacted, and new spots are generated. Add 100 ml of water to wash, then wash with saturated saline, TLC shows that there is still fluorescence at the origin. Washing with dilute potassium hydroxide aqueous solution, the raw material point is still not fully washed. Add about 8 grams of silica gel, dry-load the sample, and separate on a silica gel column. The eluent is petroleum ether: ethyl ...
Embodiment 3
[0037] Structural formula:
[0038]
[0039] Preparation of 3-isopropyl-1,3,5-cycloheptatrien-7-one-n-octadecyl ester
[0040] making process:
[0041] 2-Hydroxy-4-isopropyl-2,4,6-cycloheptatrien-1-one (3.28g, 20mmol, 1eq) and triethylamine (1~10eq) were dissolved in 100ml of dichloromethane,- 10℃~25℃, take n-octadecanoyl chloride (0.8~5eq) and dissolve it in about 30ml of dichloromethane, slowly add it dropwise into the mixed solution, then keep stirring for 3~8 hours, TLC (developing agent petroleum ether : Ethyl acetate = 5: 1) shows that a small amount of raw materials are not completely reacted, and new spots are generated. Add 100 ml of water to wash, then wash with saturated saline, TLC shows that there is still fluorescence at the origin. Washing with dilute potassium hydroxide aqueous solution, the raw material point is still not fully washed. Add about 8 grams of silica gel, dry-load the sample, and separate on a silica gel column. The eluent is petroleum ethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com