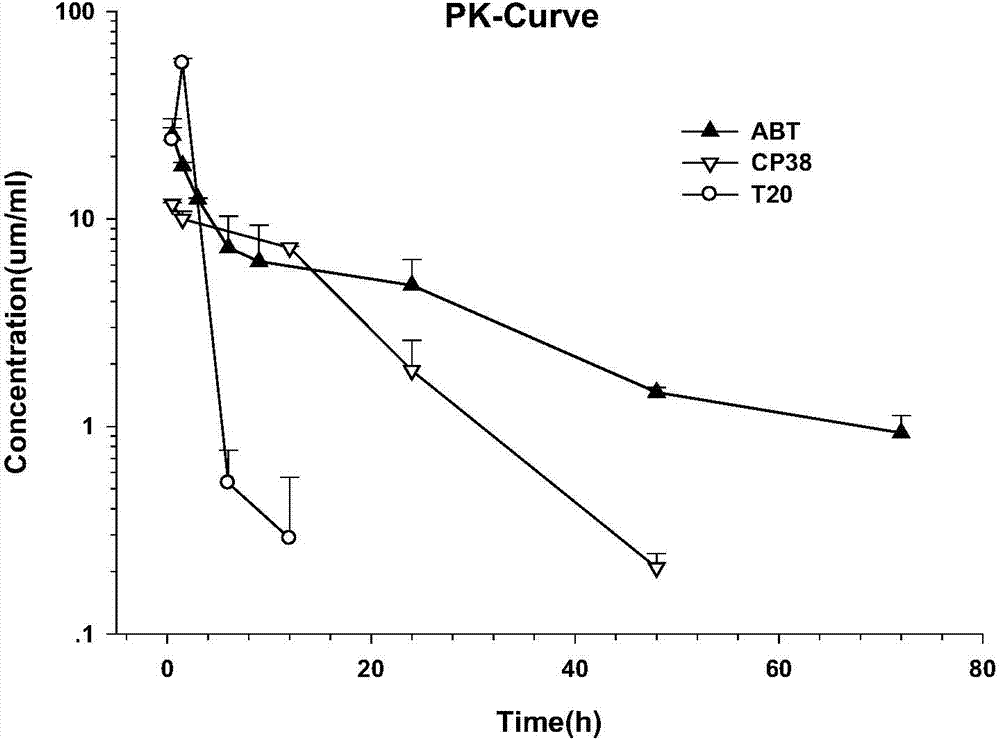
Long-acting HIV-1 membrane fusion inhibitor
A single, high-molecular technology, applied in the field of long-acting membrane fusion polypeptide drugs, can solve the problems of limited long-acting effect and loss of activity of HIV membrane fusion inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Construction and expression of ABT clones
[0033] 1.1 Materials PCR reagent: PrimeSTARDNAPolymerase (Takara) 10Xbuffer (Mg+) (Takara) dNTP (Takara)
[0034] Sterilized water: high pressure deionized water
[0035] PCR primers: synthesized by Shanghai Jieli Company
[0036] Plasmid: PHFT plasmid was donated by Beijing Huajin Ruiqing Company, PGEX‐6P‐1MD1.1‐L35‐CP38 plasmid was constructed by our laboratory Restriction enzymes: BamHI, XhoI, Bglll (Takara)
[0037] T4 ligase: purchased from Takara company
[0038] 30% Bis‐Acr polyacrylamide gel: purchased from Bio‐rad Company Ni purification column: purchased from Qiagen Company Escherichia coli HB101 and BL21(DE)3 Competent cells were purchased from Beijing Tiangen Company, other chemical reagents were domestic analysis pure.
[0039] 1.2 Experimental steps
[0040] 1.2.1 ABT clone build
[0041] In order to obtain the long-acting fusion peptide ABT (SEQ ID NO: 4), we used the method of enzymatic splicing PCR, u...
Embodiment 2
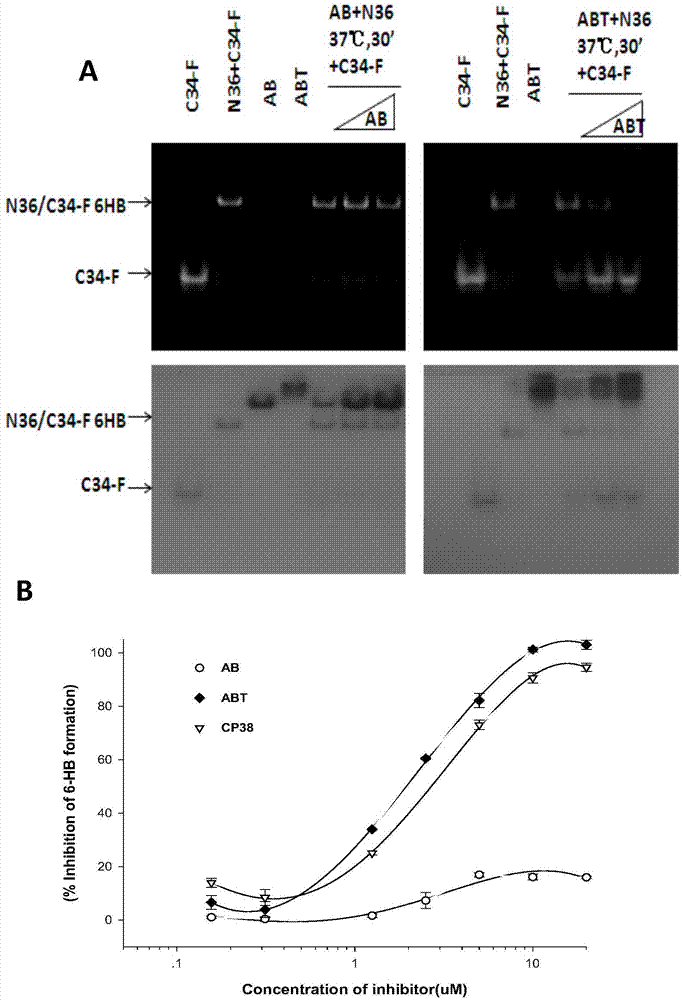
[0061] 2. FN‐PAGE detection of the effects of AB and ABT on the formation of the hexahelix
[0062] 2.1 Materials
[0063] Non-denaturing polyacrylamide gel (PAGE) gel electrophoresis kit: purchased from Beijing Tianenze Company
[0064] N36, C34, FAM‐C34 peptides were synthesized by Biosystems433A protein synthesizer
[0065] 2.2 Experimental process
[0066] (1) Prepare 18% separating gel and 5% stacking gel
[0067] (2) Prepare peptides such as N36, F‐C34, ABT, AB, N36+ABT, and N36+AB. The final concentration of each peptide is 40um, placed at 37 degrees for 30 minutes, at room temperature and protected from light, and electrophoresis 2 Hour.
[0068] (3) Fluorchem8800 (UV) detection
[0069] (4) Coomassie brilliant blue stained PAGE gel
[0070] This experiment ( figure 2 ) shows that AB does not compete with C34 or N36 to affect the formation of the six helix. Only ABT competes with C34 or N36 to affect the formation of the hexahelix.
Embodiment 3
[0071] Embodiment 3: the virus inhibition test of HIV‐1 laboratory adaptation strain and primary generation virus strain
[0072] 3.1 Experimental materials
[0073] Cells: MT‐2 cells, M7 cells Medium: 1640, 1640+10%FBS
[0074] Culture plate: 96-well flat culture plate (corning,) 96-well round bottom culture plate (corning) Cell lysate: 5% TritonX‐100
[0075] Viruses: laboratory-adapted strains HIV‐1IIIB, HIV‐1Bal virus and various HIV‐1 primary virus strains
[0076] 3.2 Experimental process
[0077] (1) Doubly dilute ABT, AB, CP38 and T20 polypeptide proteins in a 96-well plate, and set positive control wells (no polypeptide protein wells) and negative control wells (cell control wells and virus control wells).
[0078](2) Thoroughly mix the virus strain thawed at -80 degrees Celsius, and add 100 times the TCID50 value (that is, add 50% of the tissue infection dose to the well)
[0079] (3) Adjust MT‐2 or M7 cells to 1x10 5 1 / ml concentration, add 100 microliters of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com