Ganoderma sinense polysaccharide tablet and preparation method thereof
A technology for tablets and polysaccharides, applied in the field of medicine, can solve the problems of slow disintegration of Zizhi polysaccharide tablets, unable to meet the needs of treatment, moisture absorption, etc., and achieve the effects of good long-term storage stability, definite therapeutic effect, and long application history.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Zizhi polysaccharide powder:
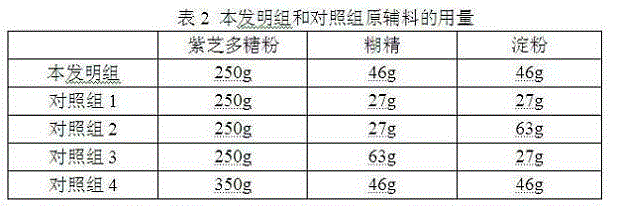
[0032] Wash the Zizhi fruiting body and / or mycelium, drain, add water and decoct twice, decoct for 2 hours for the first time, filter the medicinal liquid with a 100-mesh filter cloth, add 5 times the amount of water to the dregs for the second The second decoction for 2 hours, the medicinal liquid was filtered with a 100-mesh filter cloth, the medicinal liquid obtained from the second decoction was combined, and concentrated under reduced pressure to a clear paste with a relative density of 1.15 (heat measurement at 50°C), and 95% Ethanol until the alcohol content reaches 62%, let it stand for 24 hours, take the supernatant to recover the ethanol until it has no alcohol smell, and the precipitate after recovering the ethanol is vacuum-dried at 60°C, and the moisture content of the dry extract is controlled within 3%. Put the paste into a high-efficiency pulverizer and pulverize it into fine powder, and pass through a 100-me...
Embodiment 2
[0034] prescription:
[0035] .
[0036] Preparation:
[0037] Weigh Zizhi polysaccharide powder, starch and dextrin according to the prescription, and mix them evenly; use 30% ethanol solution to make a soft material from the mixture, and pass through a 16-mesh sieve to granulate; dry the wet granules at 60°C, so that the water content of the granules is 3.0%; Pass the dried granules through a 14-mesh sieve for granulation; add magnesium stearate to the granulated granules, mix well, and press into tablets.
[0038]
Embodiment 3
[0040] prescription:
[0041] .
[0042] Preparation:
[0043] Weigh Zizhi polysaccharide powder, starch, dextrin and sucrose according to the prescription, and mix them evenly; use 40% ethanol solution to make a soft material from the mixture, and pass through a 18-mesh sieve to granulate; dry the wet granules at 70°C, so that the water content of the granules is 3.2 %; pass the dried granules through a 16-mesh sieve for granulation; add magnesium stearate to the granules of the granules, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com