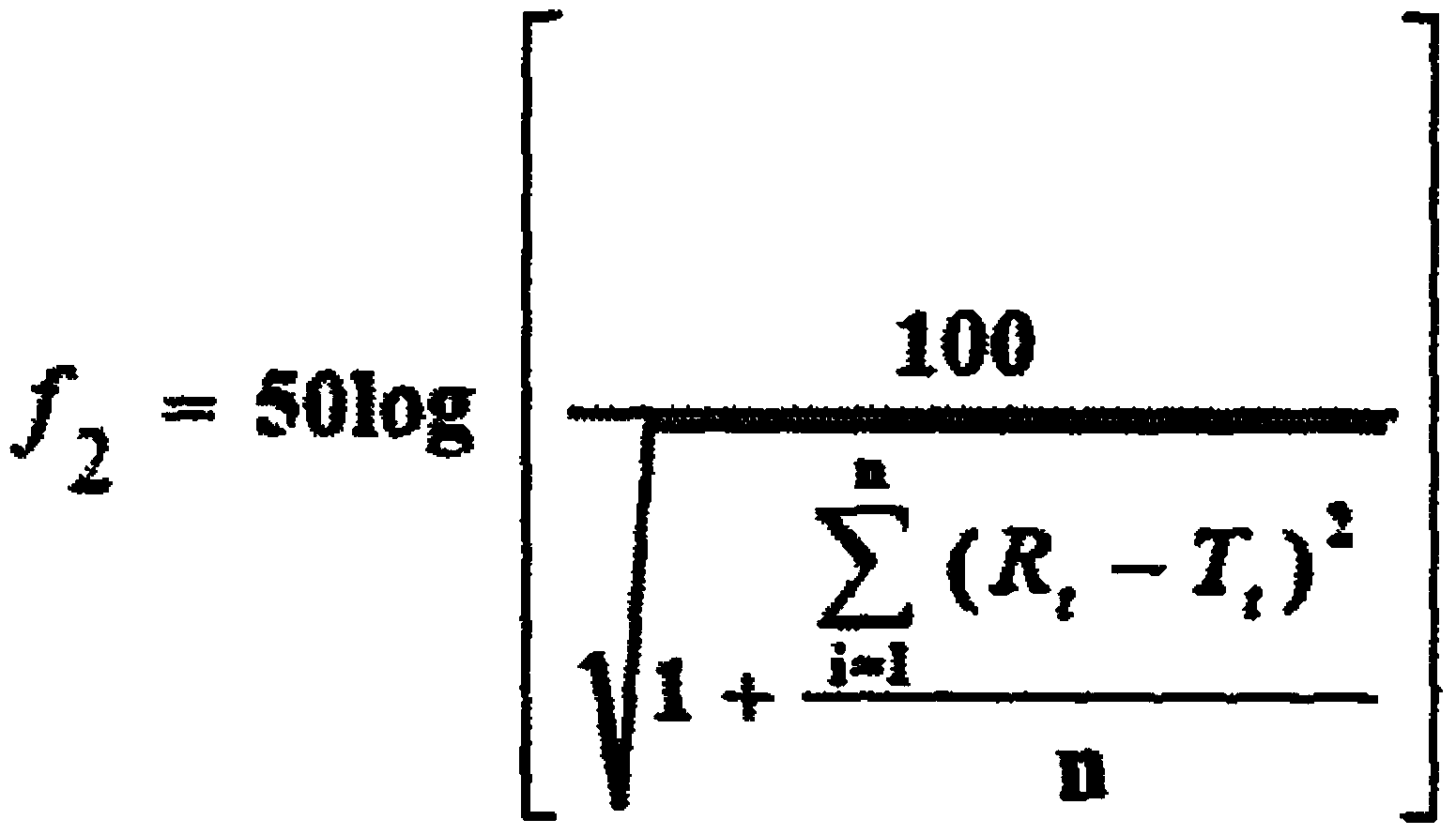Indacaterol tablets and preparation method thereof
A technology for indacaterol and preparations, applied in the field of indacaterol-containing tablets and its preparation, can solve the problems of undisclosed inclusion of indacaterol and no reminder of the proportion of components of pharmaceutical preparations, etc., to achieve The effect of content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
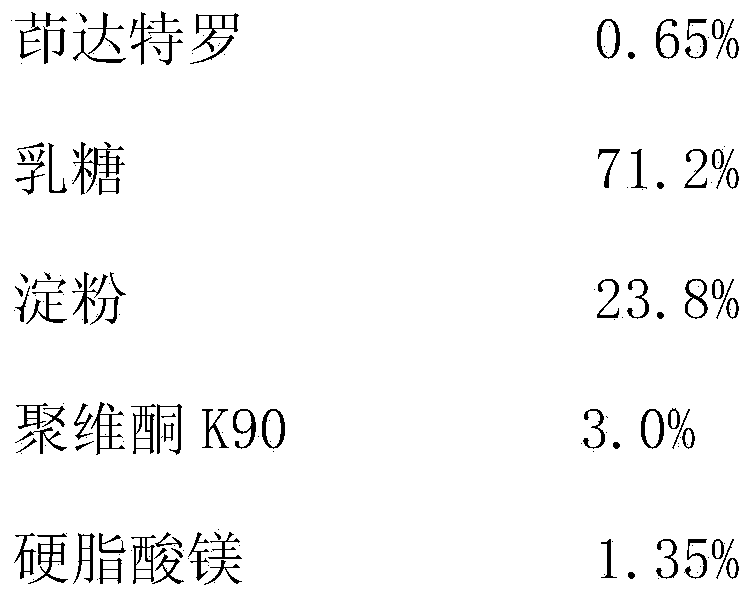
[0027] Indacaterol tablets are prepared from the following medicinal components:
[0028]
[0029] Preparation
[0030] Indacaterol and lactose are finely powdered at a ratio of 1:20, and set aside; prepare a PVP K90 aqueous solution with a concentration of 15%, and set aside; weigh the prescribed amount of indacaterol, lactose, and starch and mix evenly, add PVP K90 aqueous solution to granulate , dry, add the corresponding prescription amount of magnesium stearate and mix evenly, measure the content of the mixture, calculate the weight of the tablet, and compress the tablet to get final product.
[0031] The prepared indacaterol sheet has the following characteristics:
[0032] Content uniformity inspection: qualified. Disintegration time limit inspection: it disintegrates completely within 5 minutes, and the disintegration phenomenon is the same as that of the original preparation.
Embodiment 2
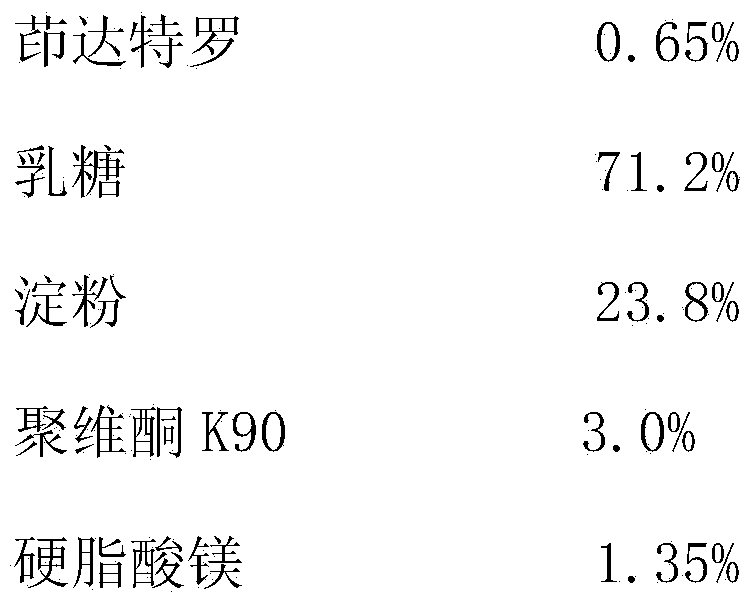
[0034] Indacaterol tablets are prepared from the following medicinal components:
[0035]
[0036] Preparation
[0037] Indacaterol and lactose are finely powdered at a ratio of 1:20, and set aside; prepare a PVP K90 aqueous solution with a concentration of 15%, and set aside; weigh the prescribed amount of indacaterol, lactose, and starch and mix evenly, add PVP K90 aqueous solution to granulate , dry, add the corresponding prescription amount of magnesium stearate and mix evenly, measure the content of the mixture, calculate the weight of the tablet, and compress the tablet to get final product.
[0038] The prepared indacaterol sheet has the following characteristics: content uniformity inspection: qualified. Disintegration time limit inspection: complete disintegration within 5 minutes.
Embodiment 3
[0040] Indacaterol tablets are prepared from the following medicinal components:
[0041]
[0042] Preparation
[0043] Indacaterol and lactose are finely powdered at a ratio of 1:20, and set aside; prepare a PVP K90 aqueous solution with a concentration of 15%, and set aside; weigh the prescribed amount of indacaterol, lactose, and starch and mix evenly, add PVP K90 aqueous solution to granulate , dry, add the corresponding prescription amount of magnesium stearate and mix evenly, measure the content of the mixture, calculate the weight of the tablet, and compress the tablet to get final product.
[0044] The prepared indacaterol sheet has the following characteristics: content uniformity inspection: qualified. Disintegration time limit inspection: complete disintegration within 5 minutes.
[0045] The specific values of the dissolution curve are shown in Table 1.
[0046] Table 1 each embodiment sample stripping curve value
[0047]
[0048]It can be seen from th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com