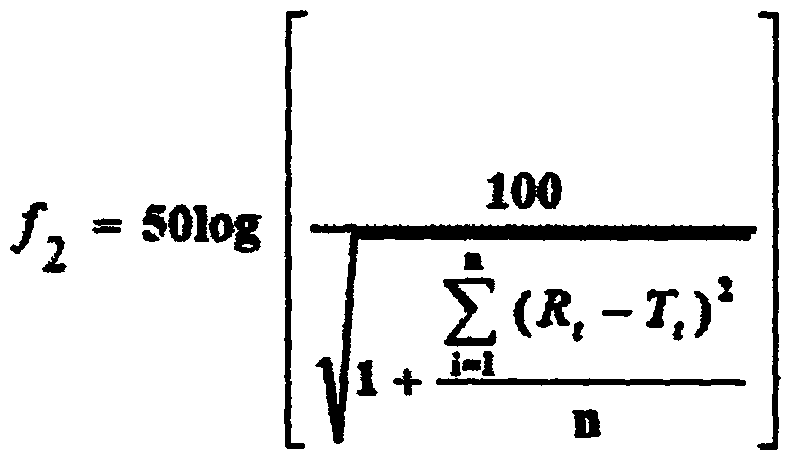Domperidone tablet and preparation method thereof
A technology for perperidone tablets and domperidone is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, which can solve the problems of slow disintegration of tablets and slow drug speed, and achieve the dissolution High, reduced equipment and operating costs, excellent appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
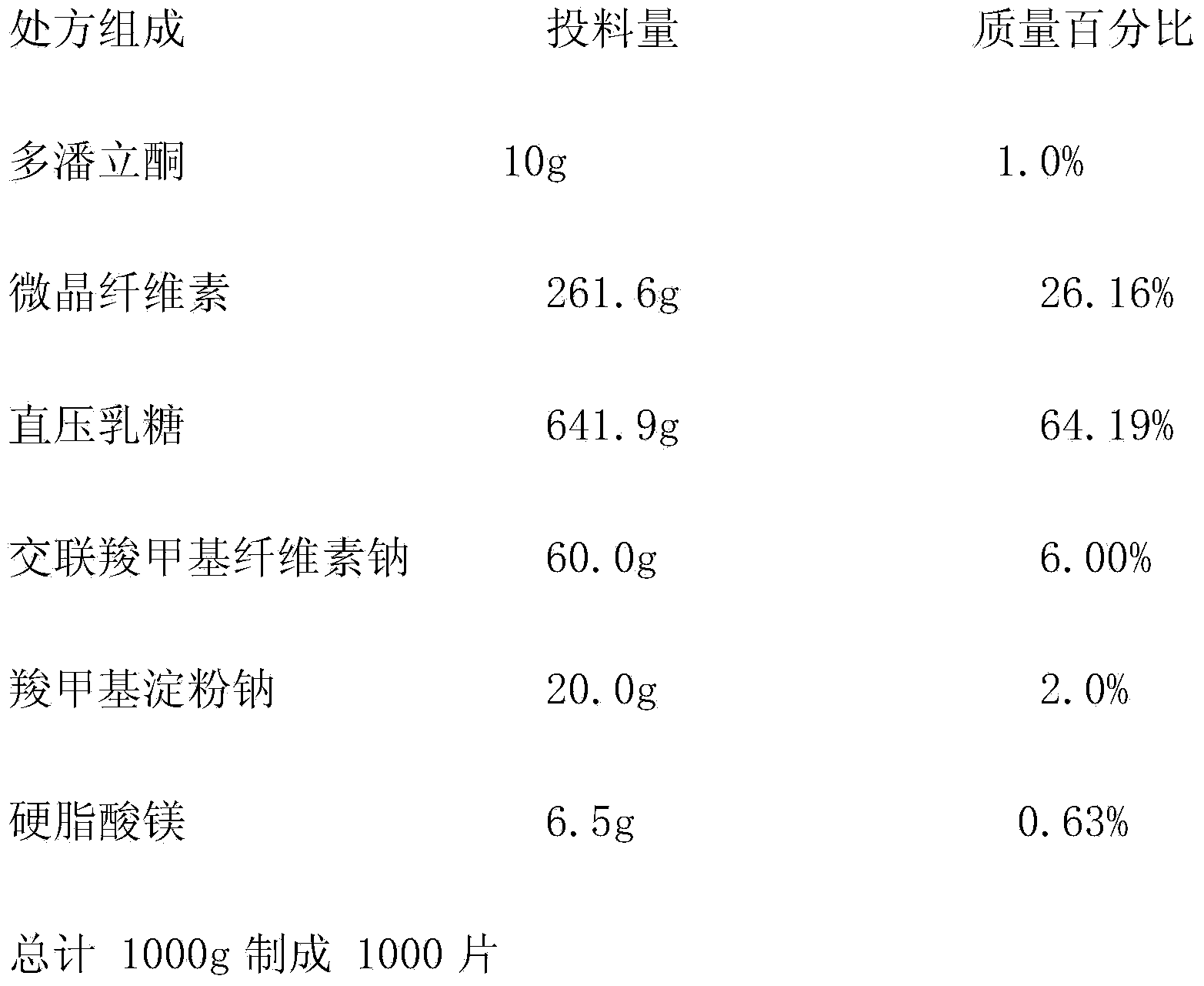
[0046]
[0047] Preparation method: the domperidone raw material is pulverized with an airflow mill, and 1000 tablets of the domperidone raw material of the prescription amount are weighed for subsequent use; the method of equal increments is adopted, and the domperidone raw material and the cross-linked sodium carboxymethyl cellulose and the prescription amount of the croscarmellose sodium and Carboxymethyl starch sodium is fully mixed, and then fully mixed with the prescribed amount of microcrystalline cellulose, direct-pressed lactose, and magnesium stearate in turn; the mixed powder is tested for bulk density and angle of repose, and the weight of the tablet should be converted according to the content. Carry out tablet compression, adjust the hardness of the tablet to 2-3kg, and the difference in filling capacity is ±5.0%.
Embodiment 2
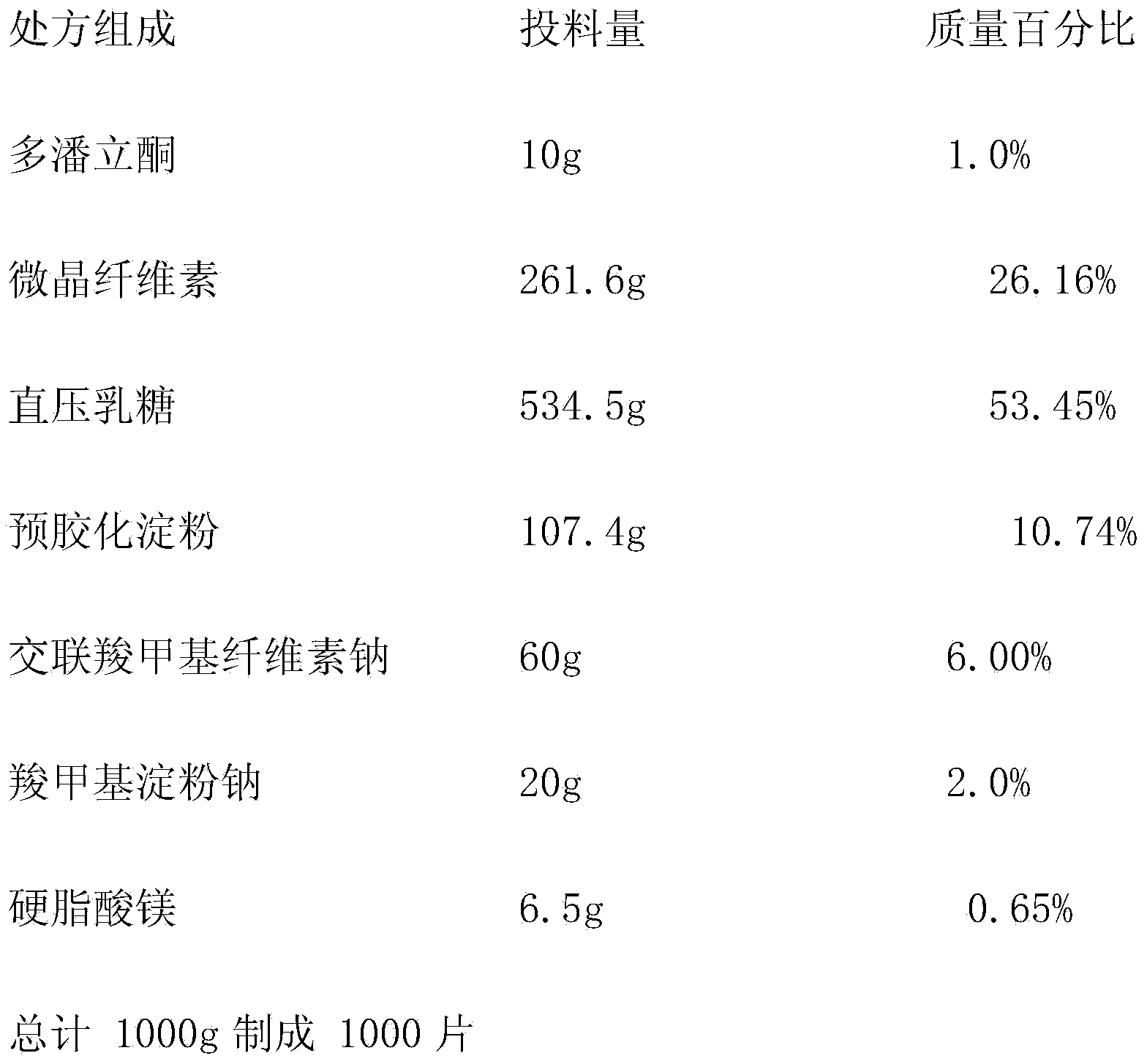
[0049]
[0050] Preparation method: the domperidone raw material is pulverized with an airflow mill, and 1000 prescription quantities of domperidone raw materials are taken for subsequent use; the equal amount of incremental method is adopted, and the domperidone raw material and the prescription quantity of croscarmellose sodium are fully Mix, and then fully mix with the prescribed amount of pregelatinized starch, sodium hydroxymethyl starch, microcrystalline cellulose, direct-compressed lactose, and magnesium stearate; after the content of the mixed material is tested, it should be converted into tablets according to the content Weight, perform tablet compression, adjust the hardness of the tablet to 2-3kg, and the difference in filling capacity is ±5.0%.
Embodiment 3
[0052]
[0053] Preparation method: pulverize the domperidone raw material with a jet mill, weigh 1000 pieces of the prescribed amount of domperidone raw material, and set aside; adopt the method of increasing the same amount, first fully mix the domperidone raw material with the prescribed amount of carboxymethyl starch sodium, and then Fully mix with the prescribed amount of pregelatinized starch, microcrystalline cellulose, direct-pressed lactose, and magnesium stearate in turn; after the mixed powder is tested for the angle of repose and bulk density content, the weight of the tablet should be converted according to the content, and the compression is carried out. Tablets, adjust the hardness of the tablets to 2-3kg, and the difference in filling capacity is ±5.0%.
[0054] The dissolution curves of the above-mentioned examples were measured respectively, and the results are shown in Table 1.
[0055] Table 1 each embodiment sample stripping curve value
[0056]
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com