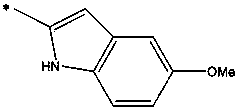
Blood stage malaria vaccine
A malaria, blood technology, applied in the direction of resistance to vector-borne diseases, antibody medical components, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0146] method
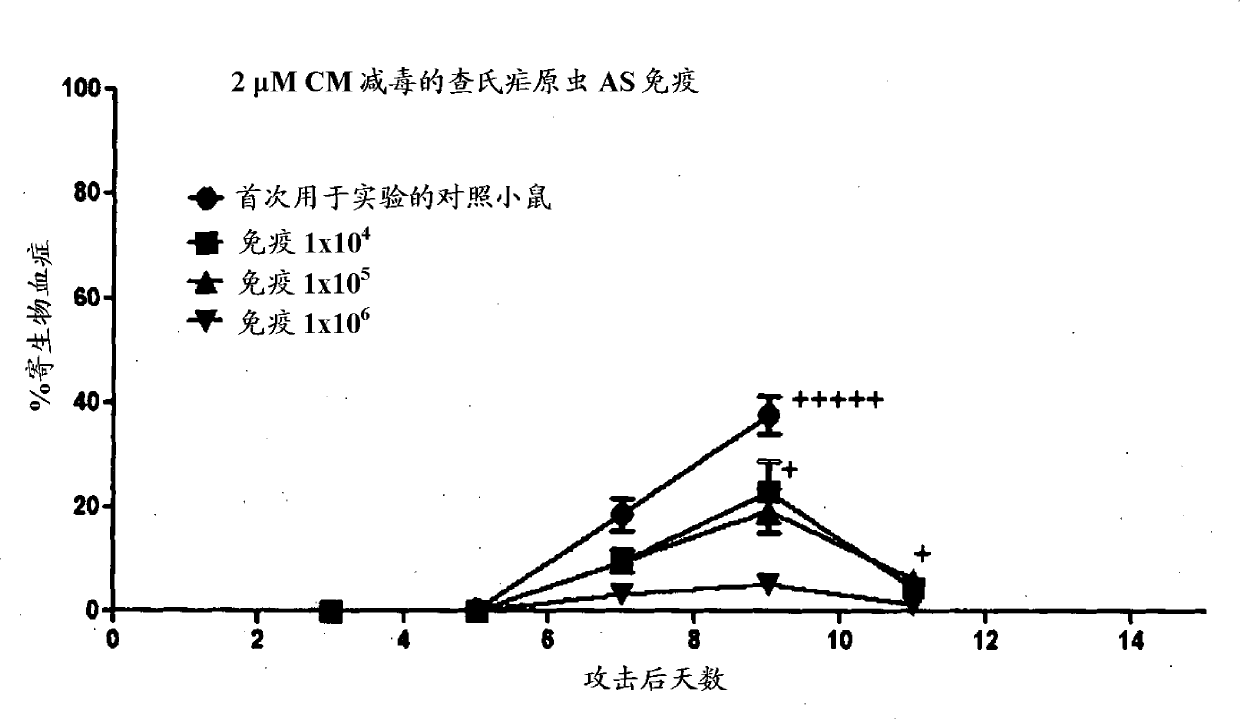
[0147] Passage mice through CO 2 They were killed by inhalation, and the parasites were harvested into the EDTA collection tube by cardiac puncture. Prepare thin blood smears to determine parasitemia.
[0148] The blood was diluted to 10 mL in serum-free IMDM (Iscoves' Modified Dulbeccos Medium). Always dilute the blood to 10% hematocrit.
[0149] Dilute tafuramycin A or centanamycin in culture medium to 20 μM from a 2 mM stock solution (in P.E.T. solution). The 2 mM stock solution diluted to 20 μM in the culture medium is necessary because the P.E.T solution causes red blood cell lysis. For 200 nM processing of red blood cells, add 50 μL of 20 μM solution for every 5 mL of blood. For 2 μM processing of red blood cells, add 500 μL of 20 μM solution for every 5 mL of blood.
[0150] The treated parasitic red blood cells (pRBC) are washed once with culture medium, and then washed with PBS once, and diluted to 5x10 in 0.9% sodium chloride 7 , 5x10 6 , 5x10 5 Or 5x10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com