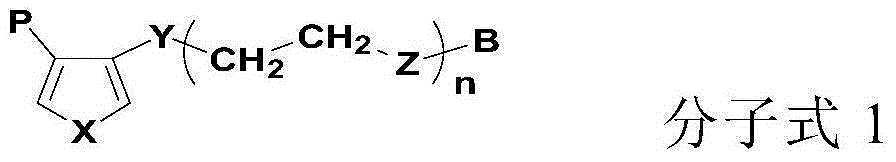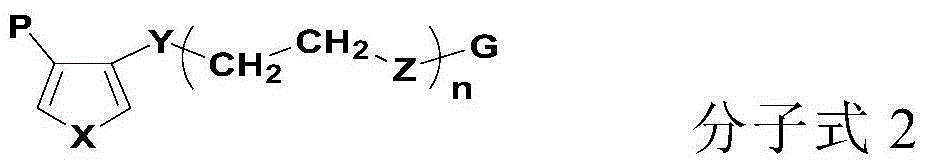Lithium-ion secondary battery and its negative pole piece
A negative pole piece and secondary battery technology, applied in secondary batteries, battery electrodes, non-aqueous electrolyte battery electrodes, etc., can solve the problems of limited improvement in the performance of lithium-ion secondary batteries and lack of lithium-ion conductivity, etc. Achieve good lithium-ion conduction performance, increase short-range conductivity, and prevent shedding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of conductive polymer
[0052] The air in a three-neck glass flask with an inner volume of 0.5 L was replaced with nitrogen, and ferric chloride (24 g) and chloroform (1 L) were added. Dissolve monomer M2 (weight average molecular weight 400, 30g), monomer M3 (weight average molecular weight 550, 10g) and monomer M1 (15g) in chloroform (300mL) and drop them into a three-necked glass flask under constant stirring middle. After stirring the mixture at room temperature for 20 h, it was added dropwise into methanol, and then the conductive polymer was separated by decanting. The conductive polymer was vacuum-dried at room temperature for 24 hours, and then vacuum-dried at 100° C. for 10 hours to obtain 36 g of conductive polymer P1 with a weight-average molecular weight of 150,000.
[0053] (2) Preparation of negative electrode sheet
[0054] The resulting conductive polymer was dissolved in NMP, and Si powder coated with carbon as the negative electrode ...
Embodiment 2
[0059] (1) Preparation of conductive polymer
[0060] The air in a three-necked glass flask with an inner volume of 0.5 L was replaced with nitrogen, and ferric chloride (27 g) and chloroform (1 L) were added. Dissolve monomer M20 (weight average molecular weight 400, 30g), monomer M5 (weight average molecular weight 400, 8g) and monomer M4 (30g) in chloroform (300mL) and drop them into a three-necked glass flask under constant stirring middle. After stirring the mixture at room temperature for 16 h, it was added dropwise into methanol, and then the conductive polymer was separated by decanting. The conductive polymer was vacuum-dried at room temperature for 24 hours, and then vacuum-dried at 100° C. for 10 hours to obtain 50 g of conductive polymer P2 with a weight-average molecular weight of 500,000.
[0061] (2) Preparation of negative electrode sheet
[0062] Dissolve the obtained conductive polymer in NMP, add carbon-coated Si powder used as negative electrode active m...
Embodiment 3
[0068] (1) Preparation of conductive polymer
[0069] The air in a three-necked glass flask with an inner volume of 0.5 L was replaced with nitrogen, and ferric chloride (27 g) and chloroform (1 L) were added. Dissolve monomer M8 (weight average molecular weight 750, 30g), monomer M7 (weight average molecular weight 600, 12g) and monomer M6 (25g) in chloroform (300mL) and drop them into a three-necked glass flask under constant stirring middle. After stirring the mixture at room temperature for 40 h, it was added dropwise into methanol, and then the conductive polymer was separated by decanting. The conductive polymer was vacuum-dried at room temperature for 24 hours, and then vacuum-dried at 100° C. for 10 hours to obtain 48 g of conductive polymer P3 with a weight-average molecular weight of 70,000.
[0070] (2) Preparation of negative electrode sheet
[0071] The obtained conductive polymer was dissolved in NMP, carbon-coated Si powder used as negative electrode active m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com