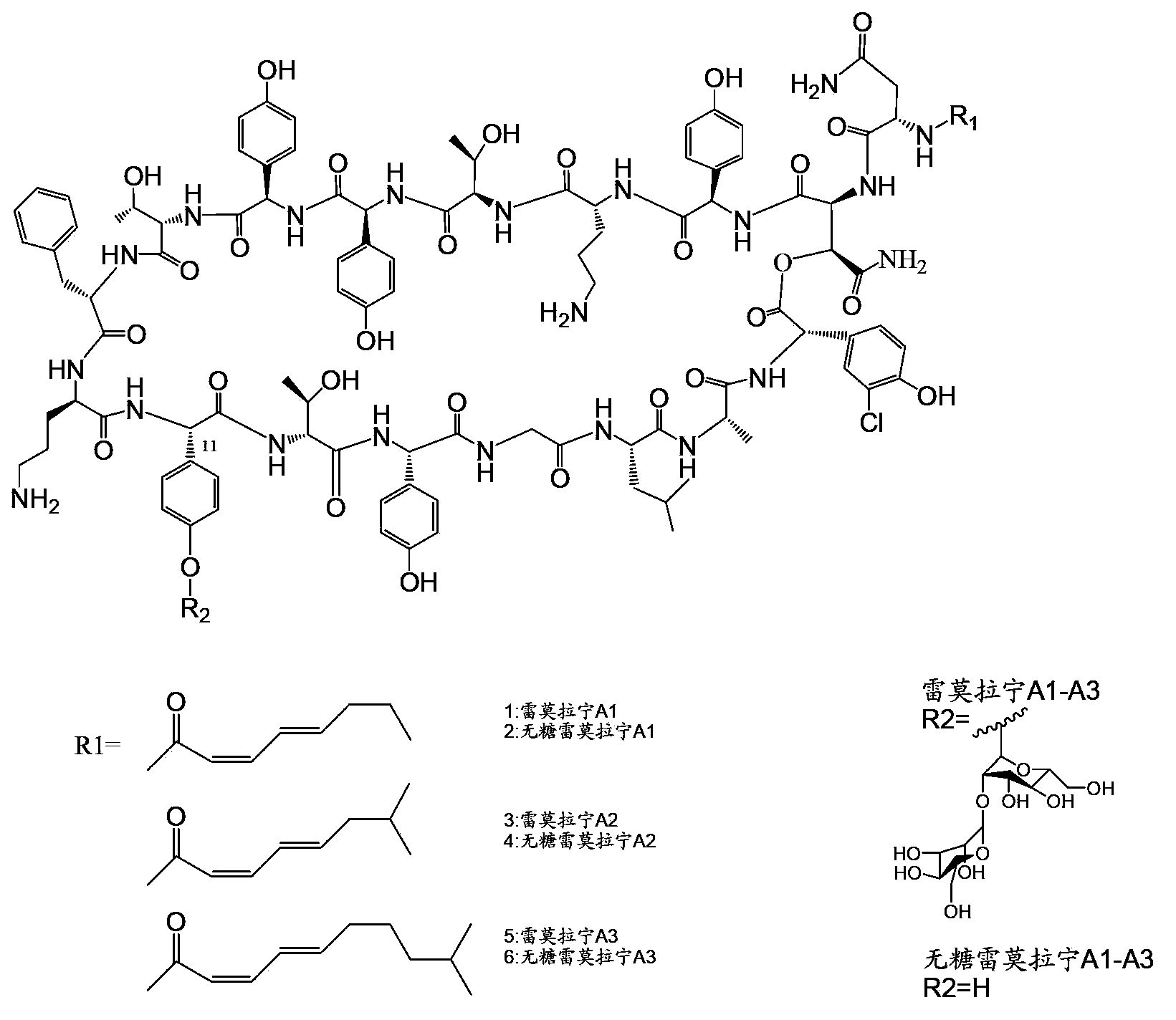
Genetic engineering bacterium for producing sugar-free ramoplanin, its construction method and application thereof
A technology of genetically engineered bacteria and genes, applied in the field of recombinant shuttle vectors and transformants, to achieve good industrialization prospects and increase stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
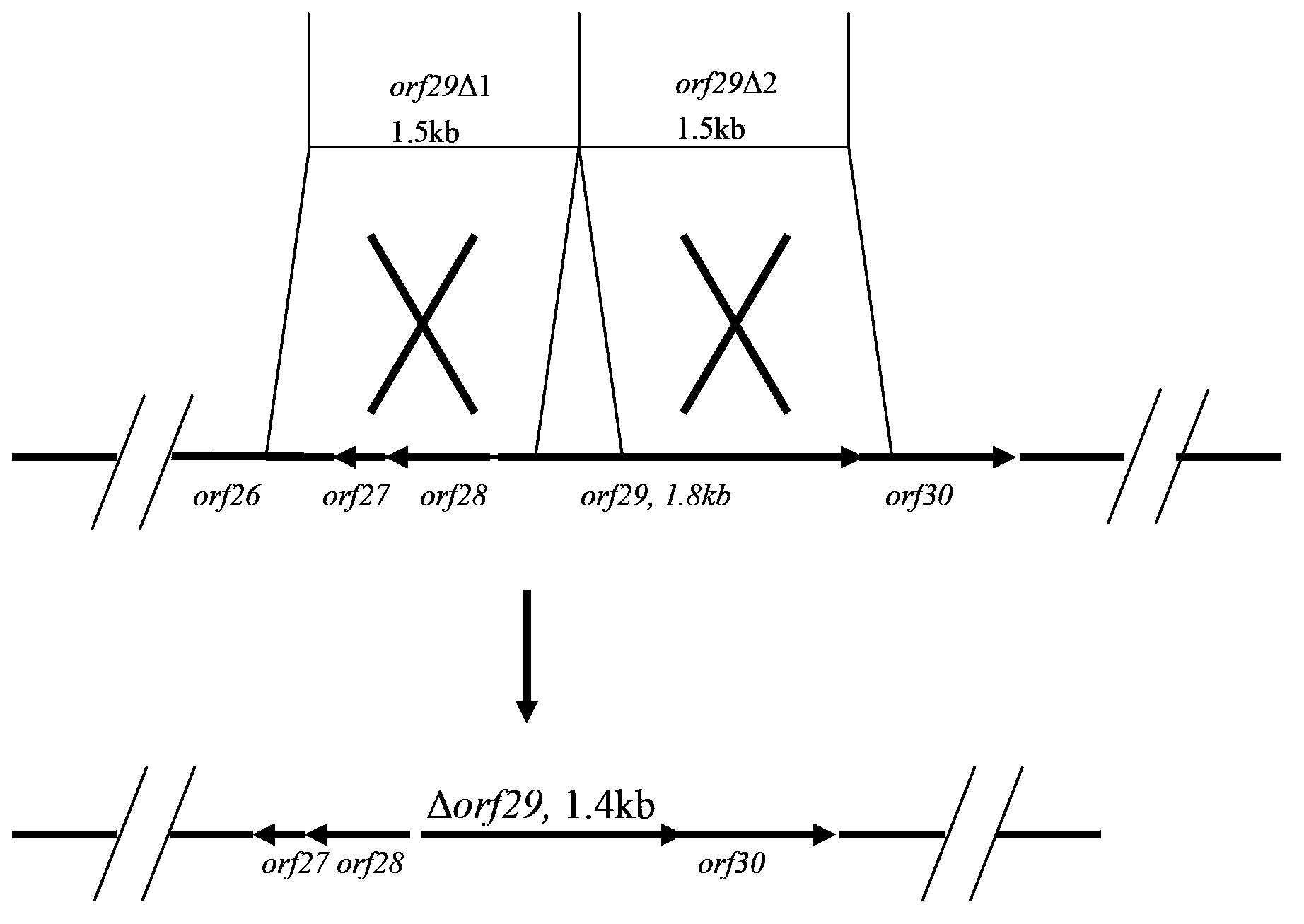
[0039] Example 1: Cloning of double exchanged left and right arms
[0040] In Actinoplanes sp.ATCC 33076, ramo-orf26, ramo-orf27 and ramo-orf28 are sequentially upstream of ramo-orf29, ramo-orf30 and ramo-orf31 are sequentially downstream of ramo-orf29, we take ramo The fragment from -orf9 upstream to ramo-orf26, the 5' end fragment of ramo-orf29 are used as the left arm of the double crossover, and the 3' end fragment of the ramo-orf29 gene to ramo-orf30 is used as the right arm of the double crossover to construct a double crossover plasmid . Firstly, primers were designed according to the published corresponding sequence of Actinoplanes sp.ATCC33076 (US 7635765):
[0041] Upstream of the left arm is 5'-AAA AAGCTT CAGGGCGATGAGGATGC-3';
[0042] Downstream of the left arm is 5'-AAA TCTAGA GAAGCCGAAGACGCG-3';
[0043] Upstream of the right arm is 5'-AAA TCTAGA GCGGTCTCGCTGCTCT-3';
[0044] Downstream of the right arm is 5'-AAA GATATC GCTGCTCGACGTAGGC-3'.
[0045] ...
Embodiment 2
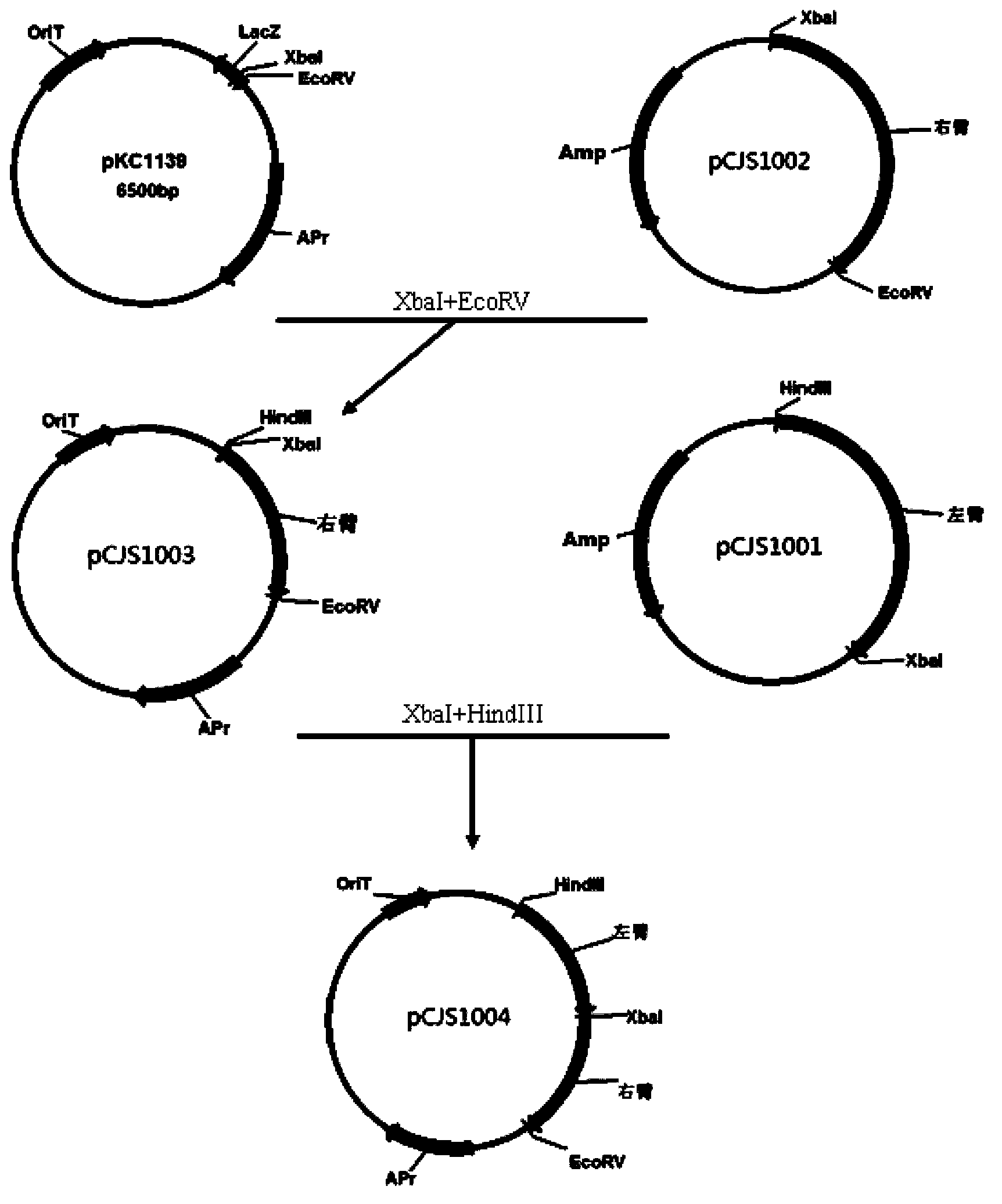
[0046] Example 2: Construction of double-exchange site-specific in-frame knockout of ramo-orf29 plasmid pCJS1004
[0047] The right arm in Example 1 was connected to the plasmid pKC1139 (purchased from Shanghai Bioengineering Technology Service Co., Ltd.), and the access sites XbaI and EcoRV were inserted to obtain the plasmid, which was named pCJS1003. Connect the left arm of Example 1 into the plasmid pCJS1003 in the forward direction, and access the sites HindIII and XbaI to obtain the plasmid. Transform the ligated product into Escherichia coli DH5α. Use the ampicillin-resistant LB plate to pick positive clones, and in LB medium After culturing overnight at 37°C, a small amount of plasmid was extracted for enzyme digestion and PCR verification, and a double-crossover in-frame knockout plasmid was finally constructed, named pCJS1004. Build a strategy map see figure 2 .
Embodiment 3
[0048] Example 3: Construction of Actinoplanes sp.ATCC 33076.D29, a sugar-free remoplanin-producing genetically engineered bacterium with knockout of glycosyltransferase
[0049] Pick an appropriate amount of bacteria from the slant of Actinomycetes mobilis ATCC 33076 and culture them in 50ml TSB medium for about 56 hours to reach the logarithmic growth phase. Transfer 1% (v / v) inoculum to 50ml TSB medium and culture them for 48 hours Let the bacteria reach the late logarithmic growth period, centrifuge and pour off the supernatant to obtain mycelia, wash the mycelium twice with 20ml LB liquid medium (4000rpm, 10min, 4°C), and finally resuspend in 20ml LB medium In, ready to use. Transform competent Escherichia coli ET12567 with pCJS1004, pick the transformants to 4ml LB medium [containing abramycin (Am) 100 μg / ml, kanamycin (Kn) 25 μg / ml, chloramphenicol (Cm) 100 μg / ml] in a small test tube, shake culture at 37°C for 12 hours. Escherichia coli ET12567 was inoculated in a 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com