Novel use of oridonin derivatives in preparing antituberculosis drugs
A technology of Rubescensine A and its derivatives, which is applied in the field of medicine and can solve the problems of no anti-tuberculosis Mycobacterium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
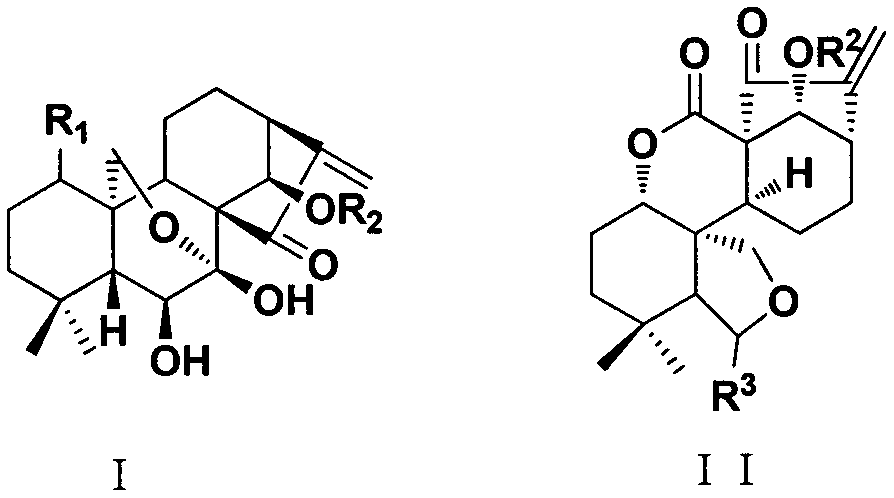
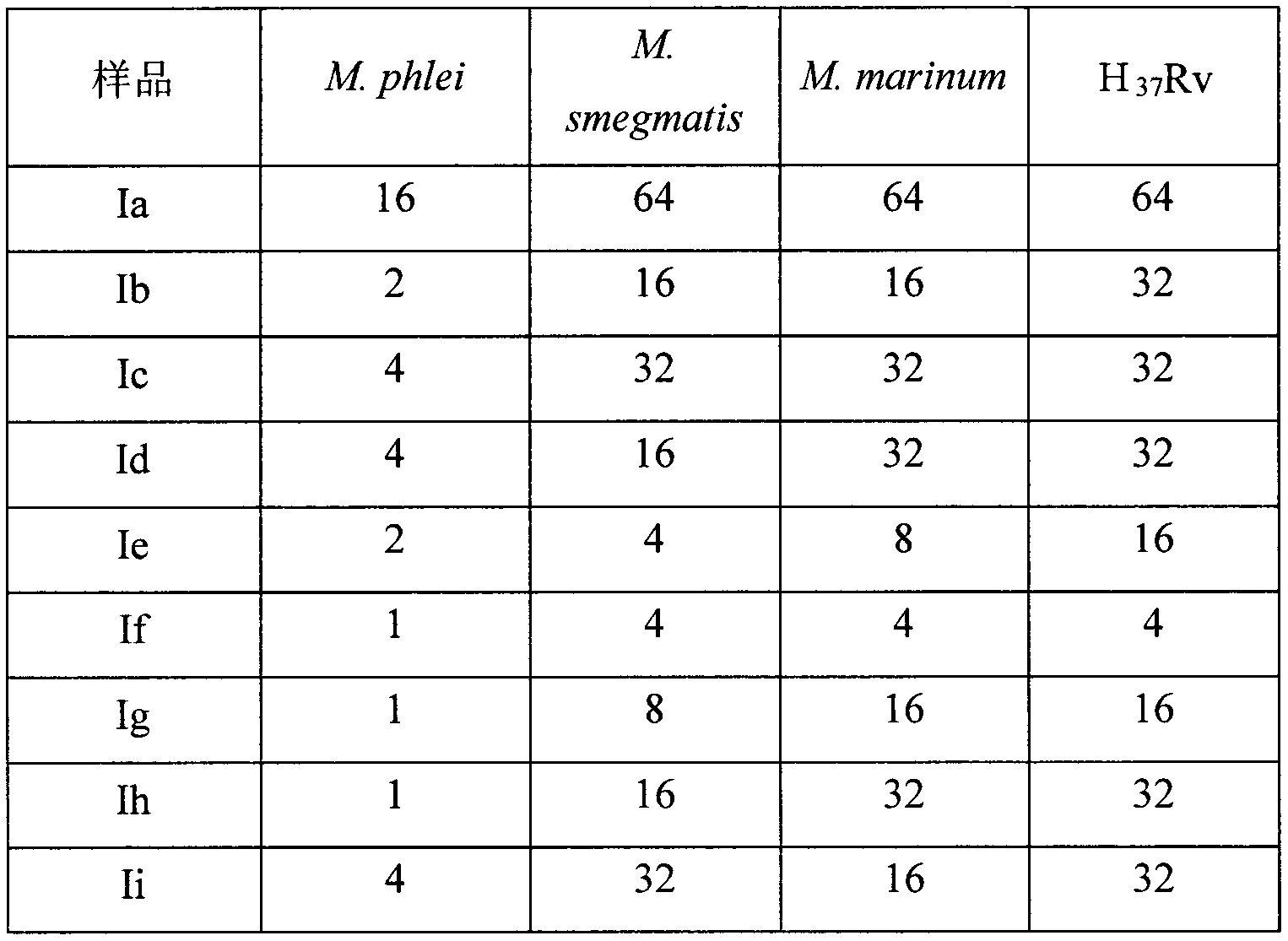
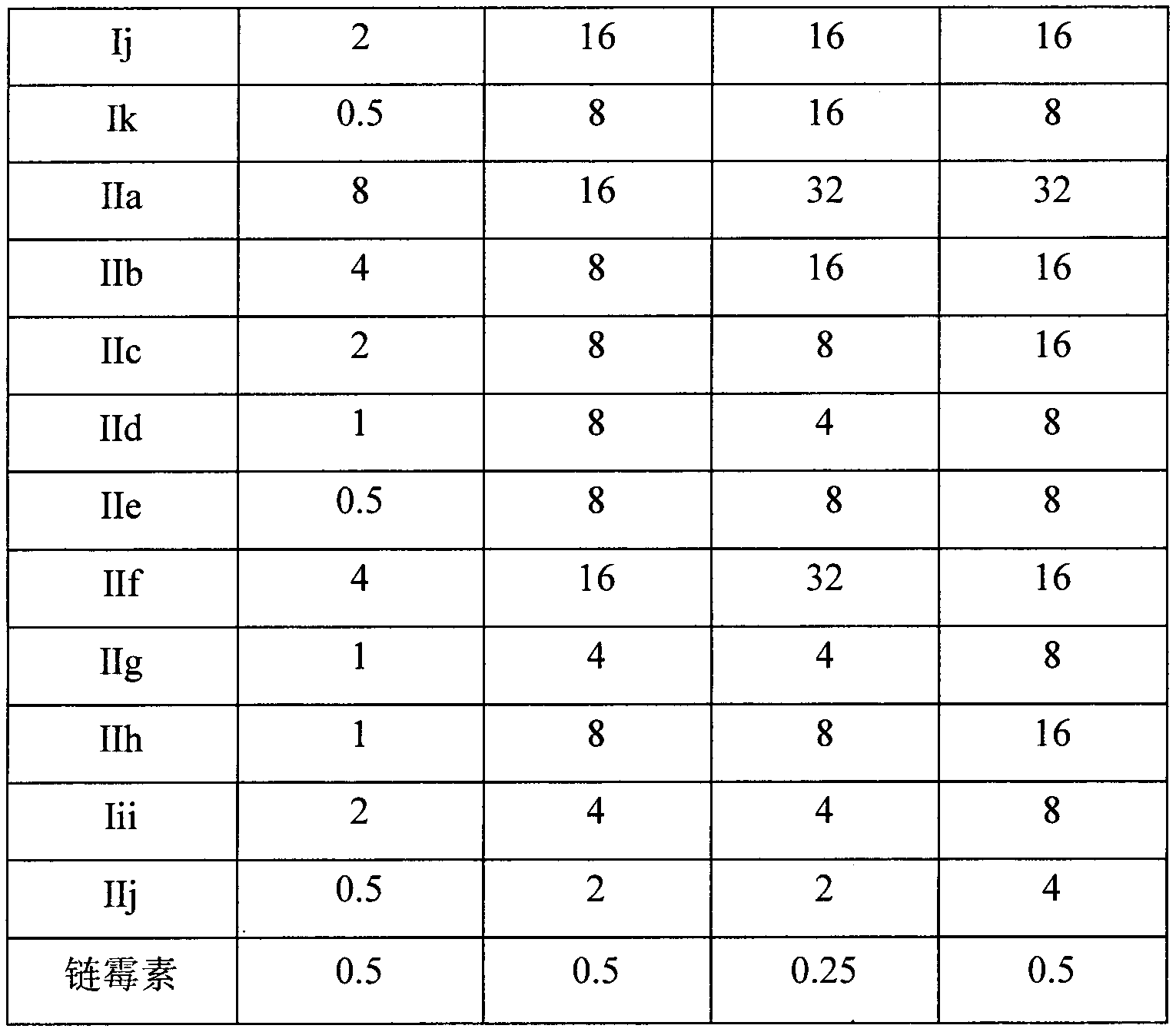
[0030] Example 1. Anti-mycobacterium tuberculosis activity experiment of oridonin
[0031] The MIC experiment was carried out in a 96-well plate. The test compound was dissolved in an appropriate amount of DMSO to make a solution with a concentration of 12.8 mg / ml, and then added to the 7H9-OADC culture medium to make a stock solution with a final concentration of 128 μg / ml. , and then diluted with liquid medium to the concentration required for the experiment. The final concentration of the tested drug was set as follows: 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125 μg / ml, a total of 11 concentration gradients.
[0032] During the detection, 100 μL of the above-mentioned drug solutions were each taken in a 96-well plate, and three groups of parallel controls were set up at the same drug dilution. Mycobacterium phlei M.phlei, Mycobacterium smegmatis M.smegmatis, Mycobacterium marinum M.marinum and Mycobacterium tuberculosis standard strain H 37 Rv was added to the culture w...
Embodiment 2
[0038] tablet
[0039]
[0040] Get above-mentioned prescription, prepare into tablet with conventional method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com