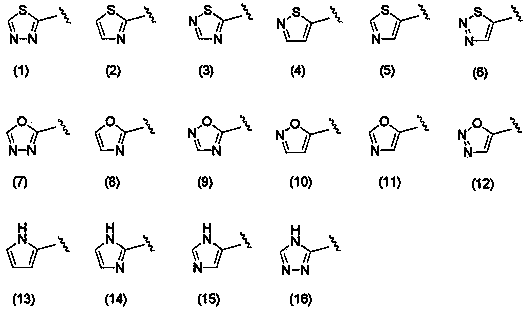
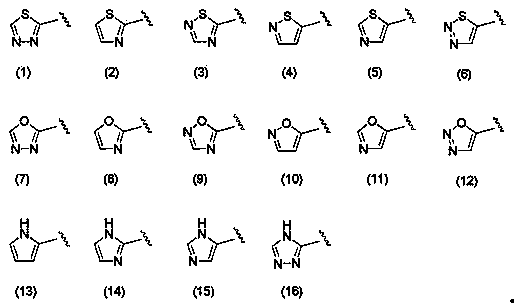
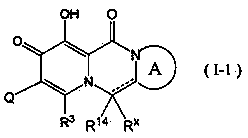
Polycyclic pyridone derivative having integrase-inhibiting activity
A compound and fusion technology, applied in the field of anti-HIV drugs, can solve problems such as cross-resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] [chemical formula 22]
[0321]
[0322] Step 1 Synthesis of Compound 3
[0323] To a solution of compound 1 (1.00 g, 2.39 mmol) in DMF (5 ml) was added compound 2 (1.72 g, 3.57 mmol) and PdCl under a stream of nitrogen 2 (dppf) (175mg, 0.239mmol) and the reaction mixture was stirred at 110°C for 1 hour. After the obtained reaction liquid was left to cool to room temperature, it was diluted with ethyl acetate (50 ml), and saturated potassium fluoride aqueous solution (50 ml) was added thereto, followed by stirring overnight. After the precipitated insoluble matter was filtered, the liquid was separated, and the aqueous layer was extracted twice with ethyl acetate. After washing the combined organic layers 3 times with water and drying over sodium sulfate, the solvent was evaporated off. The obtained crude product was purified by silica gel column chromatography. First, it was eluted with hexane-ethyl acetate (1:1, v / v), then with ethyl acetate only. The target fr...
Embodiment 2
[0329] The following compounds were synthesized by the same procedure as in Example 1.
[0330] [chemical formula 23]
[0331]
[0332] 1 H-NMR (DMSO-d 6 ) δ: 1.35 (d, J = 7.2 Hz, 3H), 1.55 (d, J = 12.6 Hz, 1H), 1.96-2.07 (m, 1H), 3.88-3.93 (m, 1H), 4.02-4.08 (m , 1H), 4.22 (s, 2H), 4.39 (dd, J = 5.7 Hz, 13.8 Hz, 1H), 4.60 (dd, J = 3.9 Hz, 13.8 Hz), 4.79-4.85 (m, 1H), 5.46- 5.49 (m, 1H), 7.04-7.11 (m, 1H), 7.21-7.28 (m, 1H), 7.46 (dd, J = 8.7 Hz, 15.6 Hz, 1H), 7.66 (s, 1H), 8.74 (s , 1H).
Embodiment 3
[0334] [chemical formula 24]
[0335]
[0336] Step 1 Synthesis of Compound 8
[0337] To a solution of compound 6 (200 mg, 0.52 mmol) in DMF (5 ml) were added triethylamine (263 mg, 2.60 mmol) and ethyl chloroformate (169 mg, 1.56 mmol) under ice-cooling. After stirring at the same temperature for 10 minutes, compound 7 (287 mg, 1.56 mmol) and dimethylaminopyridine (6 mg, 0.05 mmol) were added thereto, and then the reaction mixture was stirred at the same temperature for 1 hour. Water was added to the obtained reaction solution, which was washed three times with ethyl acetate, and the aqueous layer was extracted twice with chloroform. After drying the combined extracts over sodium sulfate, the solvent was evaporated. The obtained crude product was purified by silica gel column chromatography. First, it was eluted with chloroform only, then with chloroform-methanol (3:2, v / v). The target fraction was concentrated to give compound 8 (109 mg, yield 38%).
[0338] 1 H-NM...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap