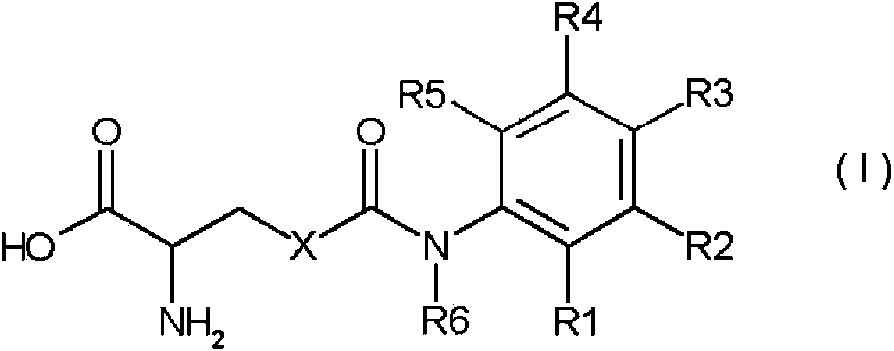
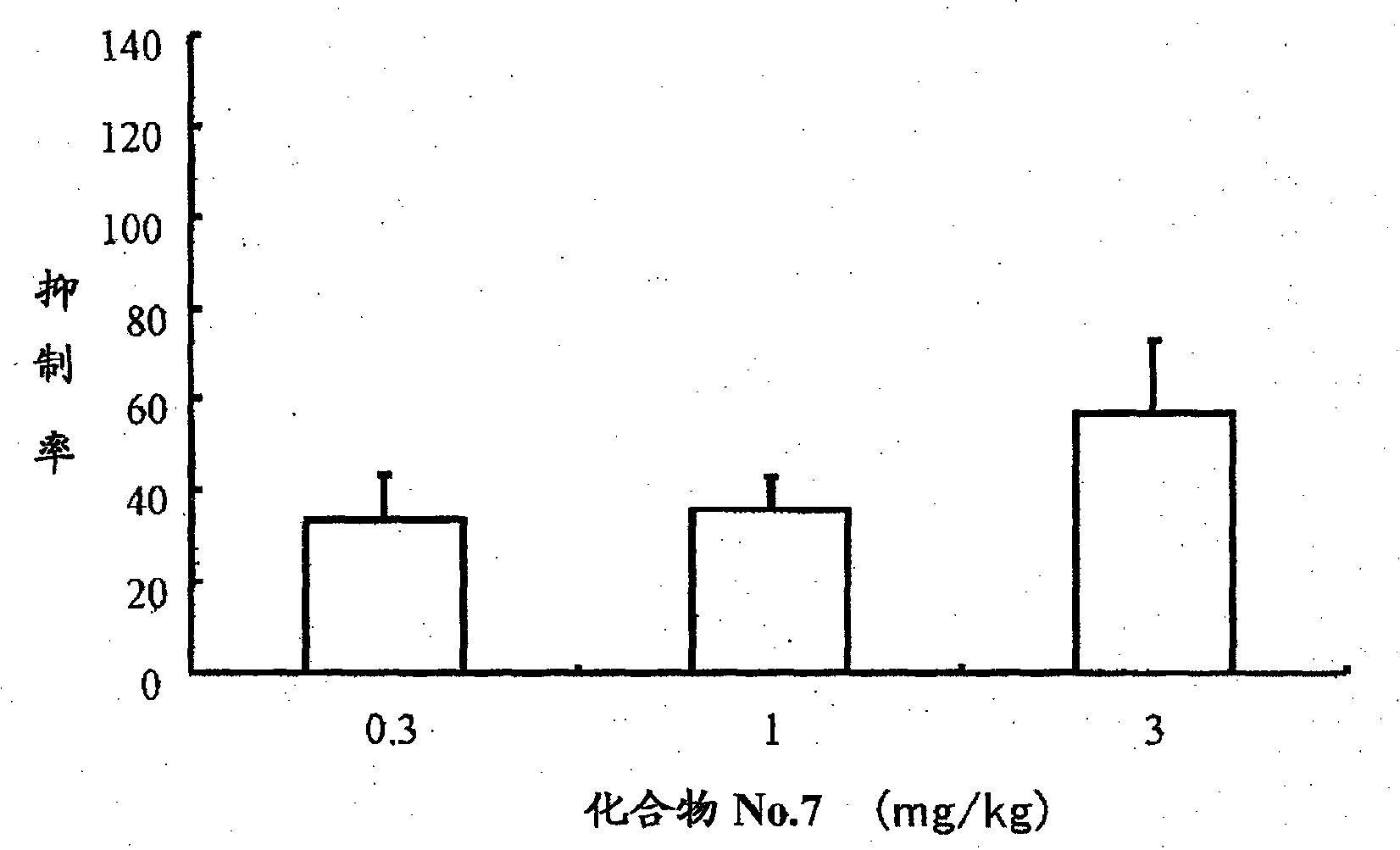CaSR agonist
A technology of substituent and carbon number, applied in the field of CaSR agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0205] Examples are given below to illustrate the present invention in more detail, but these examples do not limit the present invention.
[0206] In the present specification, the conventional method refers to a method generally used as a chemical operation represented by liquid separation operation, drying, filtration, and concentration.
[0207] In this specification, the purification step A means that the crude product obtained by a conventional method is supplied to reversed-phase high performance liquid chromatography using octadecyl chemically bonded silica gel (ODS) as a filler, and is purified with 0.1% trifluoroacetic acid ( The mixed solution of water and acetonitrile of v / v) is eluted, the method for concentrating, freeze-drying target component.
[0208] Hereinafter, the synthesis of typical compounds of the present invention shown in Table 1 will be described in more detail by citing examples, but the compounds of the present invention are not limited to these e...
Synthetic example 1
[0214] (Synthesis Example 1)N 5 Synthesis of -(3-sulfophenyl)-L-glutamine (Compound No.1)
[0215] 75mg (0.247mmol) Boc-Glu-OtBu, 112mg (0.296mmol) HATU, 41mg (0.296mmol) HOAt were dissolved in 1ml DMF, 52μl triethylamine was added, and stirred at room temperature for 10 minutes. 43 mg (0.247 mmol) of 3-sulfoaniline was added thereto, followed by stirring at room temperature overnight. After distilling off the solvent, the obtained intermediate was purified by purification step A, dissolved in 2 ml of trifluoroacetic acid, stirred at room temperature for 3 hours, and then the solvent was distilled off. The product was purified by Purification Step A to afford the title compound.
[0216] Yield: 30.8 mg (0.10 mmol), yield: 41%.
[0217] 1H-NMR (D2O, 300MHz): δ7.89(s, 1H), 7.67-7.62(m, 2H), 7.58-7.53(m, 1H), 3.99(t, 1H, J=6.4Hz), 2.73- 2.66(m, 2H), 2.33-2.25(m, 2H)
[0218] ESI(m / z): 303[M+H]+, 301[M-H]-
Synthetic example 2
[0219] (Synthesis Example 2)N 5 Synthesis of -(4-methoxy-3-sulfophenyl)-L-glutamine (Compound No.2)
[0220] Using p-anisidine-3-sulfonic acid instead of 3-sulfoaniline in Synthesis Example 1, the same operation was carried out to obtain the title compound.
[0221] Yield: 24.7 mg, yield: 23%.
[0222] 1H-NMR (D2O, 300MHz): δ7.96(s, 1H), 7.53(d, 1H), 7.04(d, 1H), 3.98(t, 1H), 3.78(s, 3H), 2.63-2.52( m, 2H), 2.30-2.05 (m, 2H)
[0223] ESI(m / z): 333[M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com