Assays For Selecting A Treatment Regimen For A Subject With Depression And Methods For Treatment
A treatment plan and technology for depression, applied in biochemical equipment and methods, microbiological determination/testing, measuring devices, etc., can solve the problem that patients have no options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1288] Example 1. Double-blind placebo-controlled study of folic acid-containing compounds in SSRI-resistant patients with major depressive disorder (MDD)
[1289] Exemplary Study Design
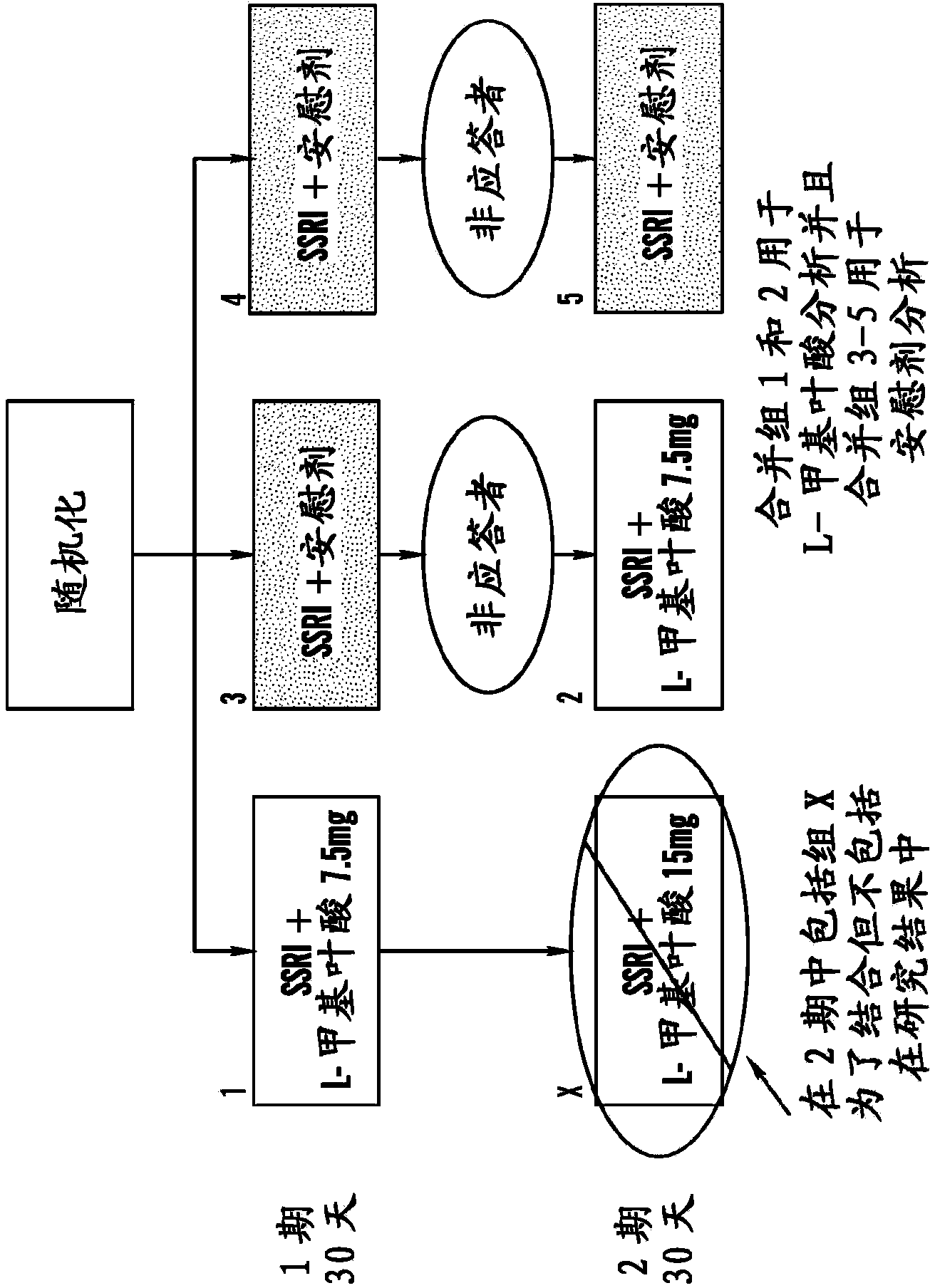
[1290] using a continuous parallel design [51] (see e.g., Figures 1A-1B and 2), a 60-day double-blind treatment of administering a folic acid-containing compound (eg, 6(S)-5-MTHF) or placebo as an SSRI adjuvant can be divided into two 30-day periods with assessments performed every 10 days. In the first phase of double-blind treatment, suitable patients can be randomized to 30 days of treatment with 6(S)-5-MTHF (15 mg / day) (n=19) or placebo (n=56), where treatment The random assignment ratios for sequential drug / drug (referring to 6(S)-5-MTHF), placebo / placebo, and placebo / drug were 2:3:3. For example only, if there is a 10% dropout rate in Phase 1, then 50 patients on placebo would complete Phase 1 of 30 days and 17 patients on 15 mg / day 6(S)-5-MTHF would complete The first phase. Patie...
Embodiment 2
[1358] Example 2 Evaluation of the efficacy of 6(S)-5-MTHF as a potentiation strategy in MDD patients (Trial 1)
[1359]A 60-day, multicenter, double-blind placebo of oral 6(S)-5-MTHF-enhancing efficacy of selective serotonin reuptake inhibitors (SSRIs) using the study design as described in the Examples and a serial parallel design A controlled study (Trial 1) has been completed in 148 patients with major depressive disorder (MDD) resistant to treatment with SSRIs. The study involved the enrollment of a total of 148 patients with MDD over 12 months across 10 medical centers or hospitals in the United States. Using a sequential parallel comparison design [51], outpatients with MDD were treated with 7.5 mg / day of 6(S)-5-MTHF or placebo as an adjuvant to SSRIs for 60 days. According to the continuous parallel design [51], the 60-day double-blind treatment was divided into two periods of 30 days each, with assessments performed every 10 days. like Figure 1A shown, during the ...
Embodiment 3
[1371] Example 3 Efficacy evaluation of 6(S)-5-MTHF as a potentiation strategy in MDD patients (trial 2)
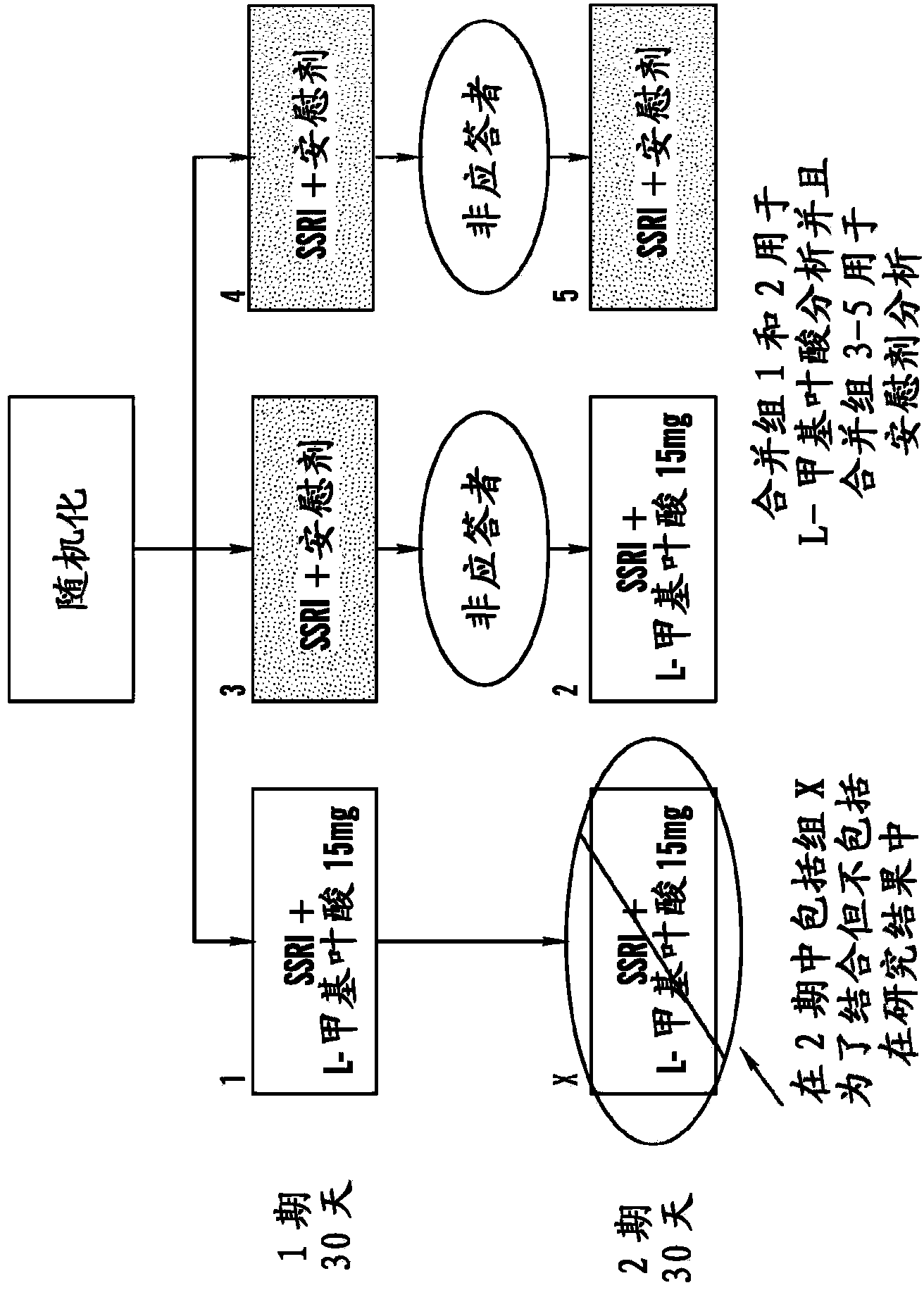
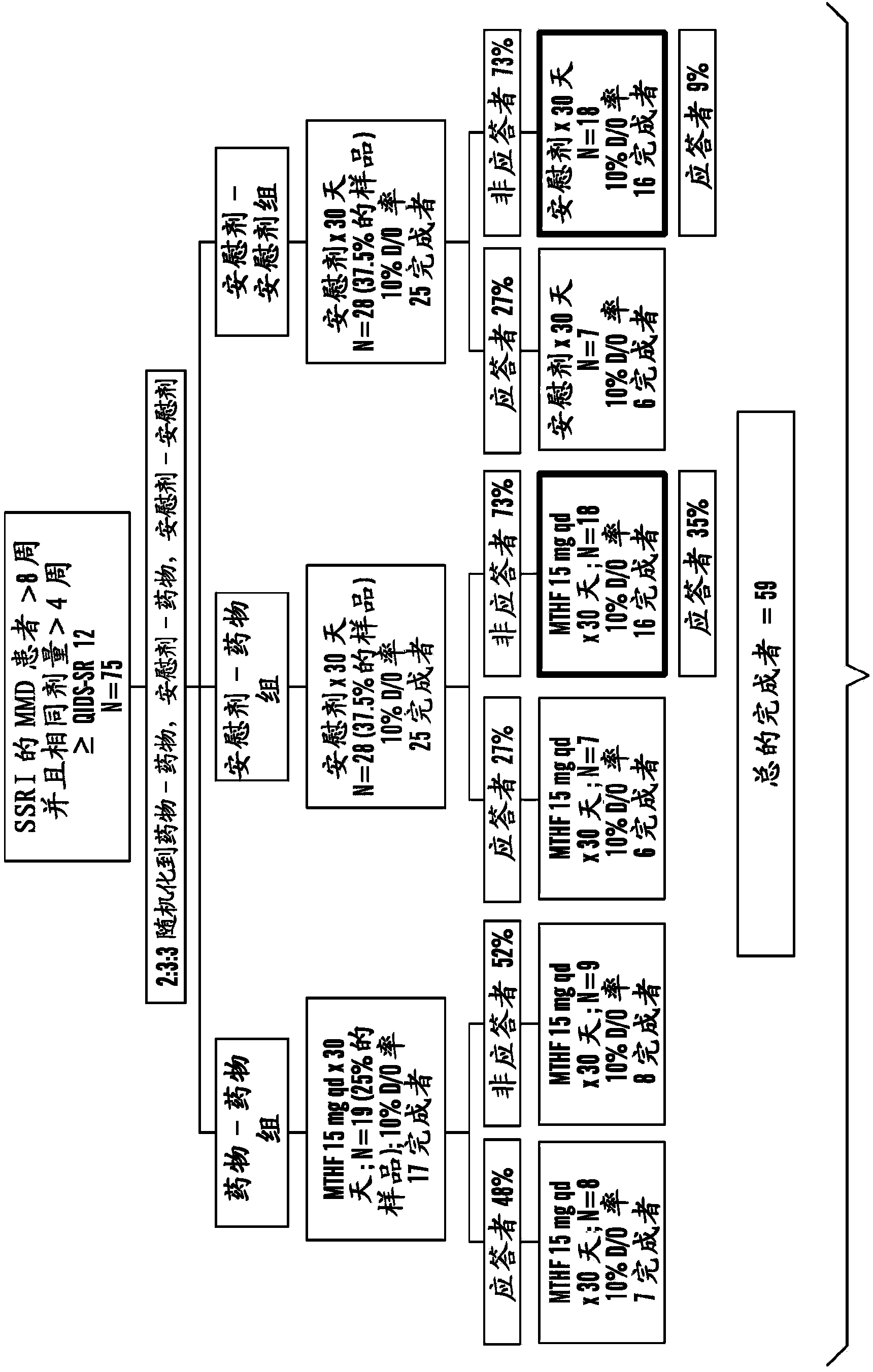
[1372] Using the study design and serial parallel design as described in Example 1, this Example 3 shows selective serotonin resistance in 75 patients with major depressive disorder (MDD) resistant to treatment with SSRIs. A 60-day, multicenter, double-blind, placebo-controlled pilot study of the enhanced efficacy of oral 6(S)-5-MTHF at higher doses (15 mg qd) of resorptive inhibitors (SSRIs) (Trial 2). Design of Trial 2 (e.g. Figure 1B shown in) as in Trial 1, except for the dosing of 6(S)-5-MTHF, which was 15 mg / drug throughout the trial for those patients assigned to the placebo-drug and drug-drug groups sky.
[1373] The key clinical objective of the study was to determine whether higher doses of oral 6(S)-5-MTHF as an adjunct to SSRIs could be compared to placebo as an adjunct to SSRIs in outpatients with MDD who were partially responding or non-responding to SSRI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com